A method and system for characterizing protein shape
A protein and characterization technology, applied in the field of analytical chemistry, which can solve the problems of lack of ability to analyze the three-dimensional structure of proteins, laborious and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
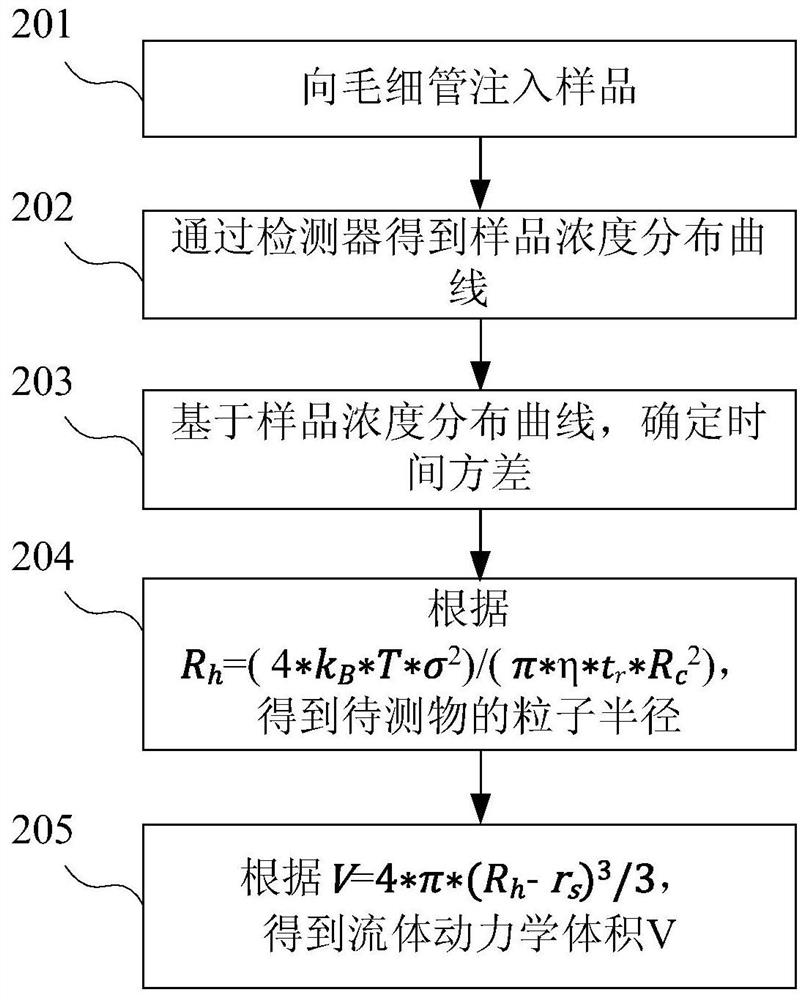
Image
Examples
Embodiment 1
[0078] In this example, the reliability of the method described in the example of the present invention is verified by characterizing the shape of seven globular proteins and one protein dimer.
[0079] Specifically, the diffusion experiments were all carried out on a Lumex CE system (model Capel 105M, St. Petersburg, Russia) equipped with a UV detector (214 nm). The sample is 3kppm insulin, cytochrome C, myoglobin, carbonic anhydrase I, α1 chymotrypsinogen, β-lactoglobulin dimer, ovalbumin, human serum albumin, no need to add neutral markers , the operating system is PH7 disodium hydrogen phosphate-citric acid buffer (5.70mL 0.2mol / L disodium hydrogen phosphate plus 14.30mL 0.1mol / L citric acid buffer), the total length of the capillary is 50cm, and the effective length is 40cm. The inner diameter is 75 μm. The separation pressure is 50mbar, the detection wavelength is 214nm, the injection pressure is 50mbar, the injection time is 5s, and the temperature is 25°C.
[0080] I...
Embodiment 2
[0087] The above-mentioned Example 1 illustrates the reliability of the method provided in the example of the present invention to characterize the protein shape. Since a change in protein conformation can lead to a change in its shape, this method can also be used to distinguish protein conformations. As a proof of concept, protein conformational changes under different pH conditions were investigated using this TDA and MS-based native method. The operating system is 3kppm lysozyme, PH7 and PH3 disodium hydrogen phosphate-citrate buffer (5.70mL 0.2mol / L disodium hydrogen phosphate plus 14.30mL 0.1mol / L citric acid buffer and 16.47mL 0.2 mol / L disodium hydrogen phosphate plus 3.53mL0.1mol / L citrate buffer). Under each voltage condition, three groups of parallel experiments were done. Explore the new method for the characterization of protein conformation changes under different pH conditions. Example 2 and Example 1 use the same experimental conditions, including the same c...
Embodiment 3
[0091] This example studies the conformational changes of BSA at different pH. As an important carrier protein and less likely to aggregate, BSA is usually used as an ideal globular protein for studying the folding and stretching behavior of proteins in chemical denaturation .
[0092] In embodiment 3, the same experimental conditions as in embodiment 2, the same TDA experimental conditions, the same mass spectrometry conditions, and the same simulation conditions are adopted. The sample uses 3kppm of BSA. Each group of experiments was repeated 3 times.
[0093] from Image 6 It can be concluded that the method provided by the embodiment of the present invention can obtain the concentration curve distribution diagram of each component on the basis of separating complex samples, and obtain the diffusion coefficient, particle radius and effective charged amount of each component.
[0094] Experimental results such as Figure 7 . according to Figure 7 It can be seen that B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com