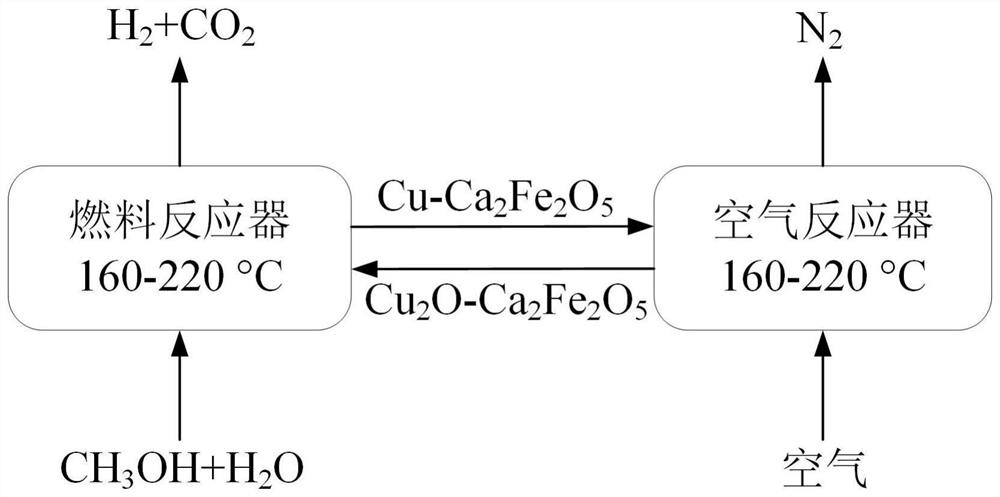
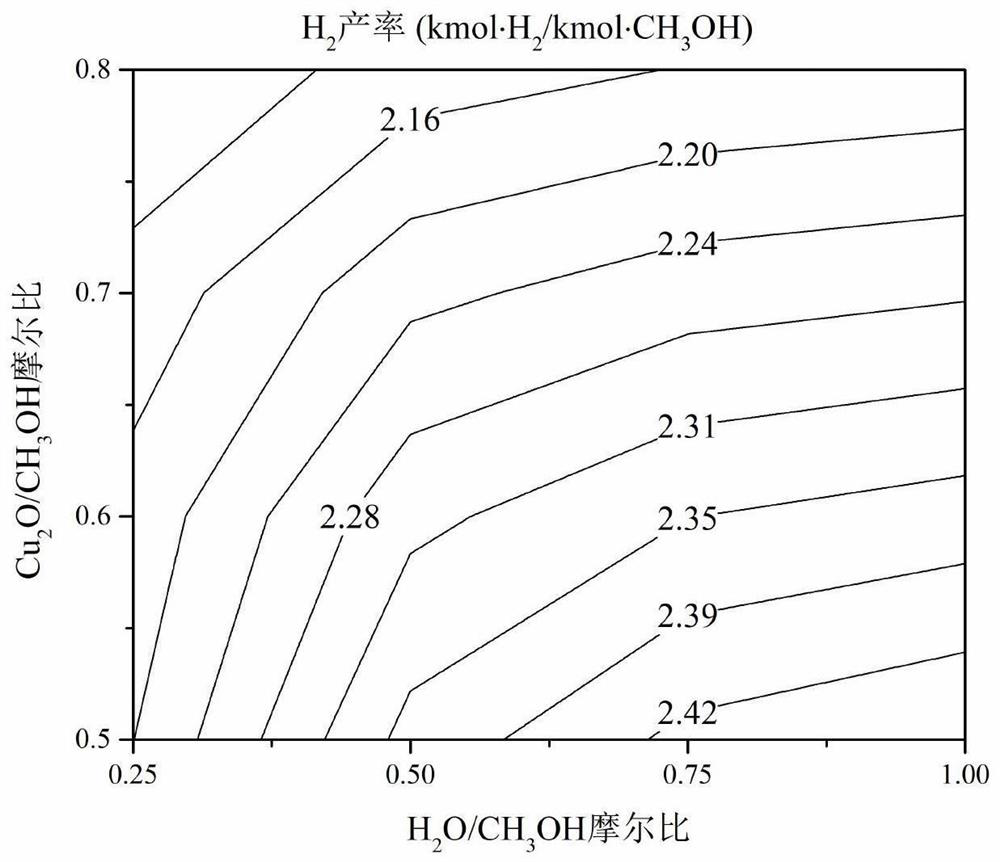
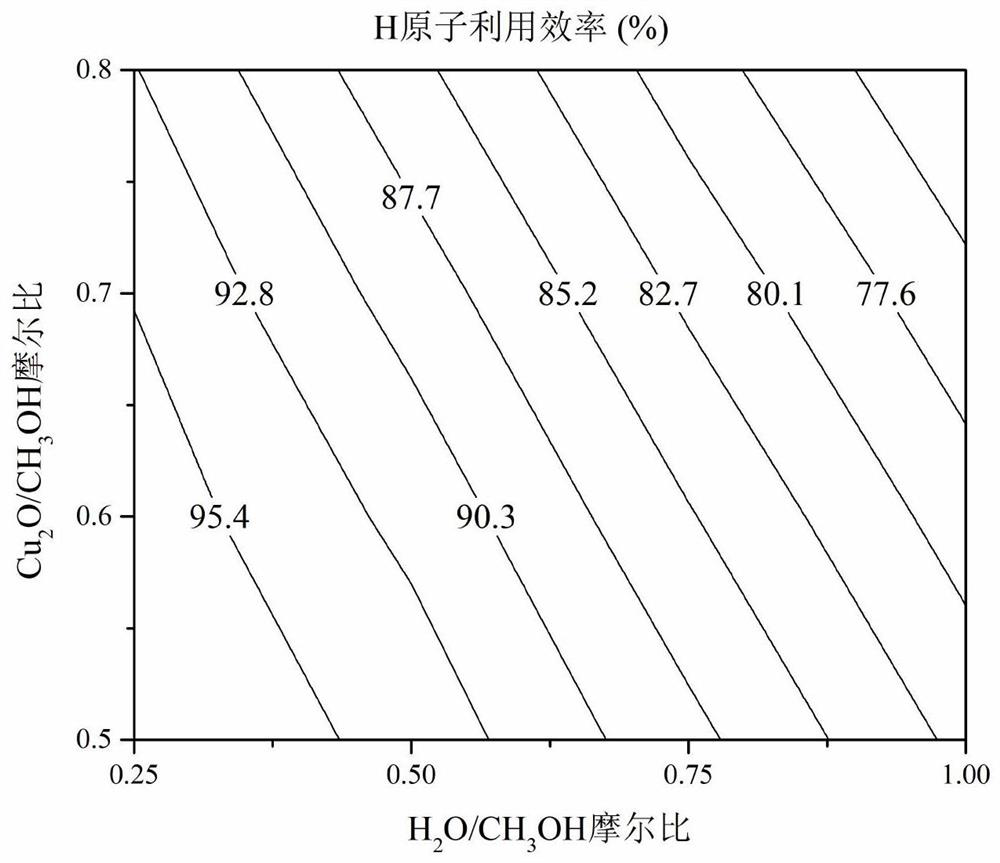
Copper-based oxygen carrier with lattice oxygen participating in methanol autothermal reforming hydrogen production, preparation and application thereof
A copper-based oxygen carrier, autothermal reforming technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, hydrogen, hydrogen/syngas production, etc., can solve the problem of high reaction temperature, unsatisfactory yield, The problem of high CO concentration in the product can achieve the effect of high hydrogen concentration, lower activation temperature, and lower CO concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0100] Example 1:
[0101] Preparation of Cu by citric acid sol-gel method combined with redox 2 O-Ca 2 Fe 2 o 5 oxygen carrier. Concrete steps are as follows: adopt copper nitrate (according to the Cu of synthesis 2 O-Ca 2 Fe 2 o 5In the oxygen carrier, the molar mass of Cu atoms accounts for 10 mol.% of the total molar mass of Cu, Fe, and Ca atoms (mixing), 3.636g (0.009mol) of iron nitrate and 2.125g (0.009mol) of calcium nitrate, dissolved in 60mL deionized In water, the molar ratio of the added amount of citric acid to the added amount of cations (copper, calcium, iron total amount) is 1.3. The solution was heated to 80° C. and kept stirring until a polymerized gel was formed, which was placed in an oven at 180° C. for 5 hours, and then calcined in a muffle furnace at 620° C. for 4 hours. After grinding the obtained solid powder, pass through methanol for deep reduction at 240°C for 20 minutes, then pass through a mixed gas of nitrogen and oxygen with an oxygen c...
Example Embodiment
[0108] Example 2:
[0109] Include the following steps:
[0110] Preparation of Cu by citric acid sol-gel method combined with redox 2 O-Ca 2 Fe 2 o 5 oxygen carrier. Concrete steps are as follows: adopt copper nitrate (according to the Cu of synthesis 2 O-Ca 2 Fe 2 o 5 In the oxygen carrier, Cu atom molar weight accounts for Cu, Fe, Ca atomic total molar weight 40mol.% ratio batching), 3.636g ferric nitrate and 2.125g calcium nitrate, dissolve in 60mL deionized water, the addition amount of citric acid and The molar ratio of the amount of cation added was 1.5. The solution was heated to 80° C. and kept stirring until a polymerized gel was formed, which was placed in an oven at 180° C. for 5 hours, and then calcined in a muffle furnace at 600° C. for 4 hours. After grinding the obtained solid powder, pass through methanol for deep reduction at 240°C for 20 minutes, then pass through a mixed gas with a concentration of 15vol.% nitrogen and oxygen at 220°C, and adjust ...
Example Embodiment
[0115] Example 3:
[0116] Include the following steps:
[0117] Preparation of Cu by citric acid sol-gel method combined with redox 2 O-Ca 2 Fe 2 o 5 oxygen carrier. Concrete steps are as follows: adopt copper nitrate (according to the Cu of synthesis 2 O-Ca 2 Fe 2 o 5 In the oxygen carrier, Cu atom molar weight accounts for Cu, Fe, Ca atomic total molar weight 30mol.% ratio batching), 3.636g ferric nitrate and 2.125g calcium nitrate, dissolve in 60mL deionized water, the addition amount of citric acid and The molar ratio of the amount of cation added was 2. The solution was heated to 80° C. and kept stirring until a polymerized gel was formed, which was placed in an oven at 180° C. for 5 hours, and then calcined in a muffle furnace at 640° C. for 4 hours. After grinding the obtained solid powder, pass through methanol for deep reduction at 240°C for 20 minutes, then pass through a mixed gas with a concentration of 12vol.% nitrogen and oxygen at 160°C, and adjust th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap