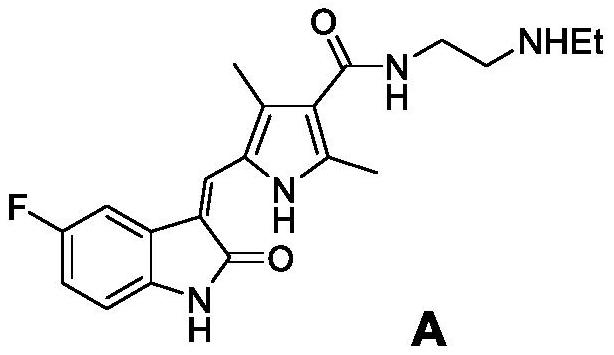
Method for controlling impurities difficult to remove in synthesis of antitumor drug sunitinib
An anti-tumor drug, sunitinib technology, applied in the field of synthesis of anti-tumor pharmaceutical compounds, can solve the problems of difficult removal and high cost, and achieve the effect of shortening the synthesis cycle, simple process and reducing the cost of impurity removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
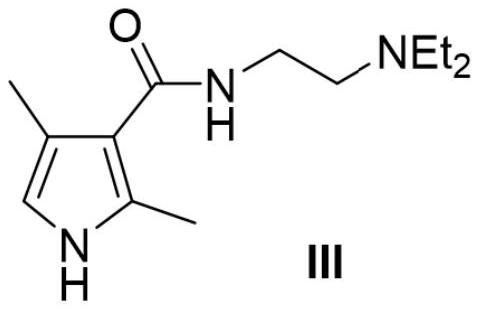
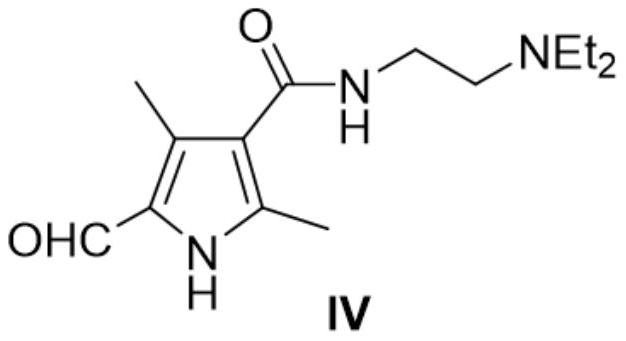
[0040] Preparation of Intermediate IV
[0041]
[0042] Proceed as follows:
[0043] All the following operations were carried out under dark conditions,
[0044] (1) Add 500ml of DCM and 59.6g of DMF into the reaction flask, cool down to -5°C, and add oxalyl chloride dropwise. After the dropwise addition was completed, stirring was continued at room temperature for 2 h to obtain solution A.
[0045] (2) Cool down to -20°C-30°C and add dropwise 600ml of DCM solution containing 146.9g of intermediate III to solution A. After dropping, continue to stir until the reaction is complete to obtain solution B.
[0046] (3) Add 1L of water to solution B, separate the water phase, adjust the pH to 12 with 40wt% KOH (260ml), and obtain a precipitate by filtration;
[0047] (4) The precipitate was dissolved with ethyl acetate to obtain a solution. The solution was washed with water and saturated brine respectively, then dried over anhydrous magnesium sulfate, and filtered to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com