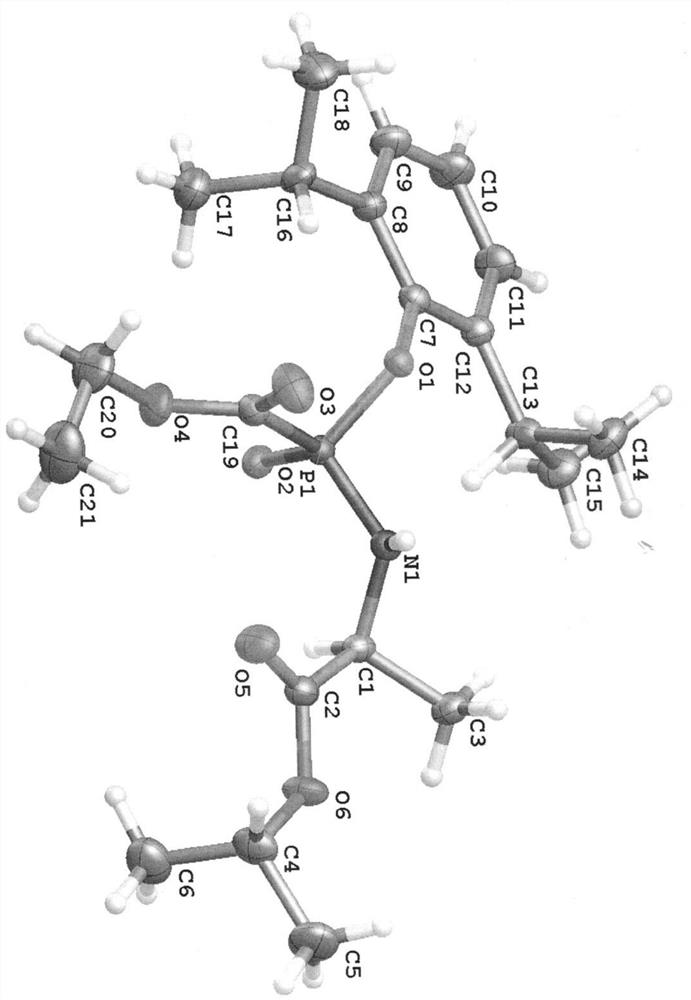
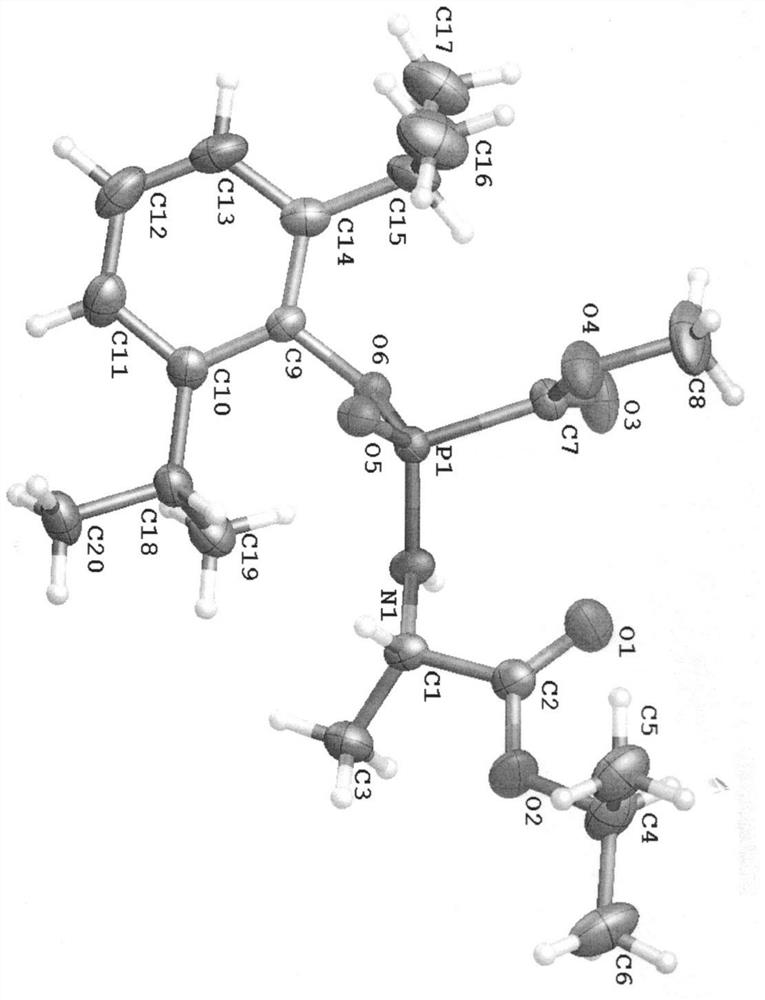
A kind of propofol phosphoramide derivative, its preparation method and its application in medicine
A pharmacy and drug technology, applied in the fields of propofol phosphoramide derivatives and intermediates and preparations thereof, can solve the problems of poor water solubility and poor stability of propofol, and achieve the effect of good oral absorption characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (S)-2-[[(R)-(2,6-Diisopropyl)phenoxy-ethoxycarbonyl-phosphoryl]amino]propanoic acid isopropyl ester (1)
[0062] (S)-isopropyl 2-[[(R)-(2,6-diisopropylphenoxy)-(ethoxycarbonyl)phosphoryl]amino]propanoate(1)
[0063]
[0064]
[0065] first step
[0066] Ethoxycarbonylphosphonic acid (1b)
[0067] Ethoxycarbonylphosphonic acid
[0068] Ethyl diethoxyphosphoroformate 1a (210.0 g, 1.0 mol) was dissolved in 800 mL of acetonitrile, trimethylbromosilane (460.0 g, 3.0 mol) was added dropwise, and reacted at 65°C for 2 hours. After the reaction, it was concentrated under reduced pressure to obtain ethoxycarbonylphosphonic acid 1b as a yellow liquid (154.0 g, 1.0 mol, yield 100%).
[0069] second step
[0070] Ethyl dichlorophosphoroformate (1c)
[0071] Ethyl dichlorophosphorylformate
[0072] Dissolve methoxycarbonylphosphonic acid 1b (154.0g, 1.0mol) in 650mL of dichloromethane, under nitrogen protection, add oxalyl chloride (510.0g, 4.0mol) dropwise at 0°C, DMF (...
Embodiment 2
[0090] (S)-2-[[(R)-(2,6-diisopropyl)phenoxy-methoxycarbonyl-phosphoryl]amino]propanoic acid isopropyl ester (2)
[0091] (S)-isopropyl 2-[[(R)-(2,6-diisopropylphenoxy)-(methoxycarbonyl)phosphoryl]amino]propanoate(2)
[0092]
[0093]
[0094] first step
[0095] Methyl diethoxyphosphoroformate (2b)
[0096] Methyldiethoxyphosphorylformate
[0097] Triethyl phosphite 2a (50.0 g, 0.3 mol) was dissolved in 250 mL of dichloromethane, methyl chloroformate (28.4 g, 0.3 mol) was added dropwise at 0°C, and slowly raised to room temperature to react overnight. After the reaction, it was concentrated under reduced pressure to obtain methyl diethoxyphosphoroformate 2b as a colorless liquid (59.0 g, 0.3 mol, yield 100%).
[0098] second step
[0099] Methoxycarbonylphosphonic acid (2c)
[0100] Methoxycarbonylphosphonic acid
[0101] Methyl diethoxyphosphoroformate 2b (59.0 g, 0.3 mol) was dissolved in 250 mL of acetonitrile, trimethylbromosilane (138.2 g, 0.9 mol) was added d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com