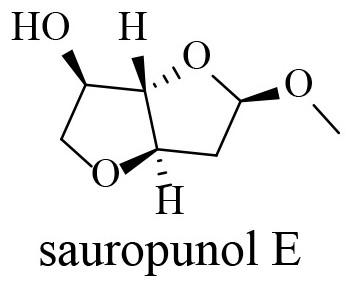
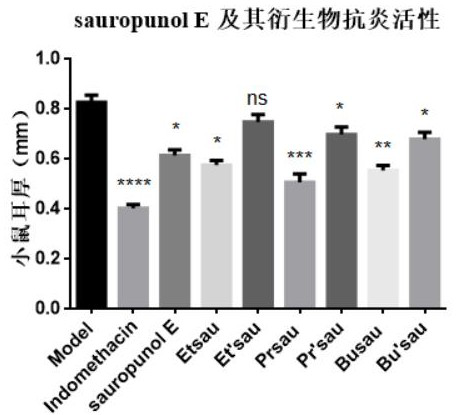
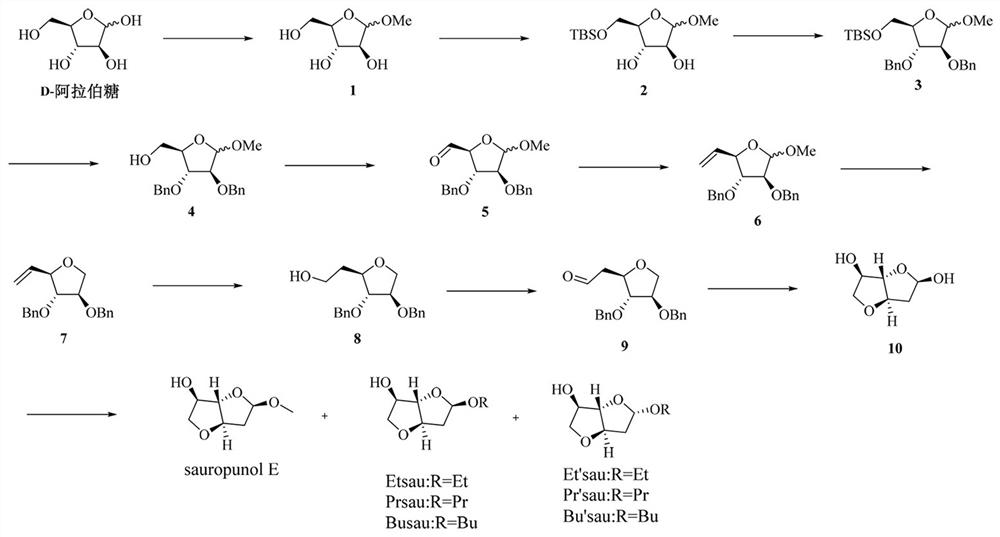
Preparation method of compound with 3,4-trans-3,6-anhydrohexfuranose structure
A technology of hexofuranose and compounds, applied in the direction of organic chemistry methods, organic chemistry, drug combination, etc., can solve the problems that cannot be formed directly, achieve the effect of solving the source problem and enriching the structure-activity relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: D-arabinose (10g, 66.7mmol) was dissolved in 50mL of dry methanol, and chloroacetyl (0.01mL, 0.14mmol) was slowly added dropwise under ice-bath conditions, and the temperature was naturally raised to room temperature after stirring, and then continued Stir for 4 hours, add sodium bicarbonate powder to the reaction solution to adjust the pH value to 7, filter, concentrate under reduced pressure, and separate by silica gel column chromatography (dichloromethane:methanol volume ratio is 50:1) to obtain compound No.1 (10.5g, 64.0 mmol, 96%).
[0032] Dissolve compound NO.1 in dry N,N - Dimethylformamide (DMF) 150mL, stirring, 0 ℃ ice bath for 30min, sequentially add imidazole (11g, 172mmol), tert-butyldimethylsilyl chloride (TBSCl, 12g, 80mmol), after stirring for 10h, add Quenched with ice water, CH 2 Cl 2 (dichloromethane) extracted three times, then washed five times with ice water, washed three times with saturated sodium chloride, concentrated under redu...
Embodiment 2
[0035] Example 2: Dissolve compound NO.2 (8.7g, 31.3mmol) in 80 mL of N,N dimethylformamide, add sodium hydride (60%, 3.8g, 93.9mmol) under ice-water bath conditions, and Stirring under this condition for 30 min, adding benzyl bromide (8.2 mL, 9 mmol), and stirring for 5 h after naturally warming to room temperature. After the reaction was completed, quenched with ice water, extracted three times with a mixed solvent of ethyl acetate:petroleum ether=1:1, after the organic phases were combined, washed three times with ice water, twice with saturated sodium chloride solution, and decompressed Concentrate, and perform silica gel column chromatography (petroleum ether: ethyl acetate = 50:1) to obtain compound NO.3 (13.2 g, 92.3%) as a yellow oil.
[0036] α-anomer: [α] D 25 = -28(c = 2.1 in CHCl 3 ); 1 H-NMR (300 MHz, CDCl 3 ): δ7.51 – 7.25 (m, 10H), 4.79 – 4.53 (m, 5H), 4.25 – 4.10 (m, 2H), 4.10 – 3.98(m, 1H), 3.86 – 3.67 (m, 2H), 3.43 (d, J = 9.2 Hz, 3H), 0.97 (d, J =...
Embodiment 3
[0037] Example 3: Dissolve compound NO.3 (13.2g, 28.8mmol) in 30mL of tetrahydrofuran, add 1M tetrabutylammonium fluoride (9.1g, 28.8mmol), stir at room temperature for 8h, after the reaction, add methanol Quenched, concentrated under reduced pressure, and subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 50:1-10:1) to obtain colorless oily compound NO.4 (8.92g, 90%).
[0038] α-anomer: [α] D 25 = +19.6 (c = 1.5 in CHCl 3 ); 1 H-NMR (300 MHz, CDCl 3 ):δ 7.35 (dd, J = 5.5, 2.3 Hz, 10H), 4.96 (s, 1H), 4.65 – 4.49 (m, 4H), 4.16 (dt, J = 6.6, 3.2 Hz, 1H), 4.01 (dt, J = 8.8, 2.7 Hz, 2H), 3.85 (dd, J =12.0, 2.9 Hz, 1H), 3.66 (dd, J = 11.6, 3.5 Hz, 1H), 3.40 (s, 3H); 13 C-NMR (75MHz, CDCl 3 ): δ138.5, 127.6, 127.7, 127.4, 110.2, 84.0, 82.0, 81.2, 73.3,63.6, 55.8,, 26.3, 25.8; HRMS(ESI) m / z Calcd. for C 25 h 32 NaO 8 [M+Na]+: 367.1603, found 367.1605.
[0039] β-anomer: [α] D 25 = -28 (c = 2.1 in CHCl 3 ); 1 H NMR (300 MHz, CD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com