Percutaneous absorption type preparation
A preparation and adhesive technology, which can be used in respiratory diseases, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of reducing skin adhesion characteristics, short-term efficacy, difficulties, etc. Absorption characteristics, excellent transdermal absorption rate, excellent efficacy period effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
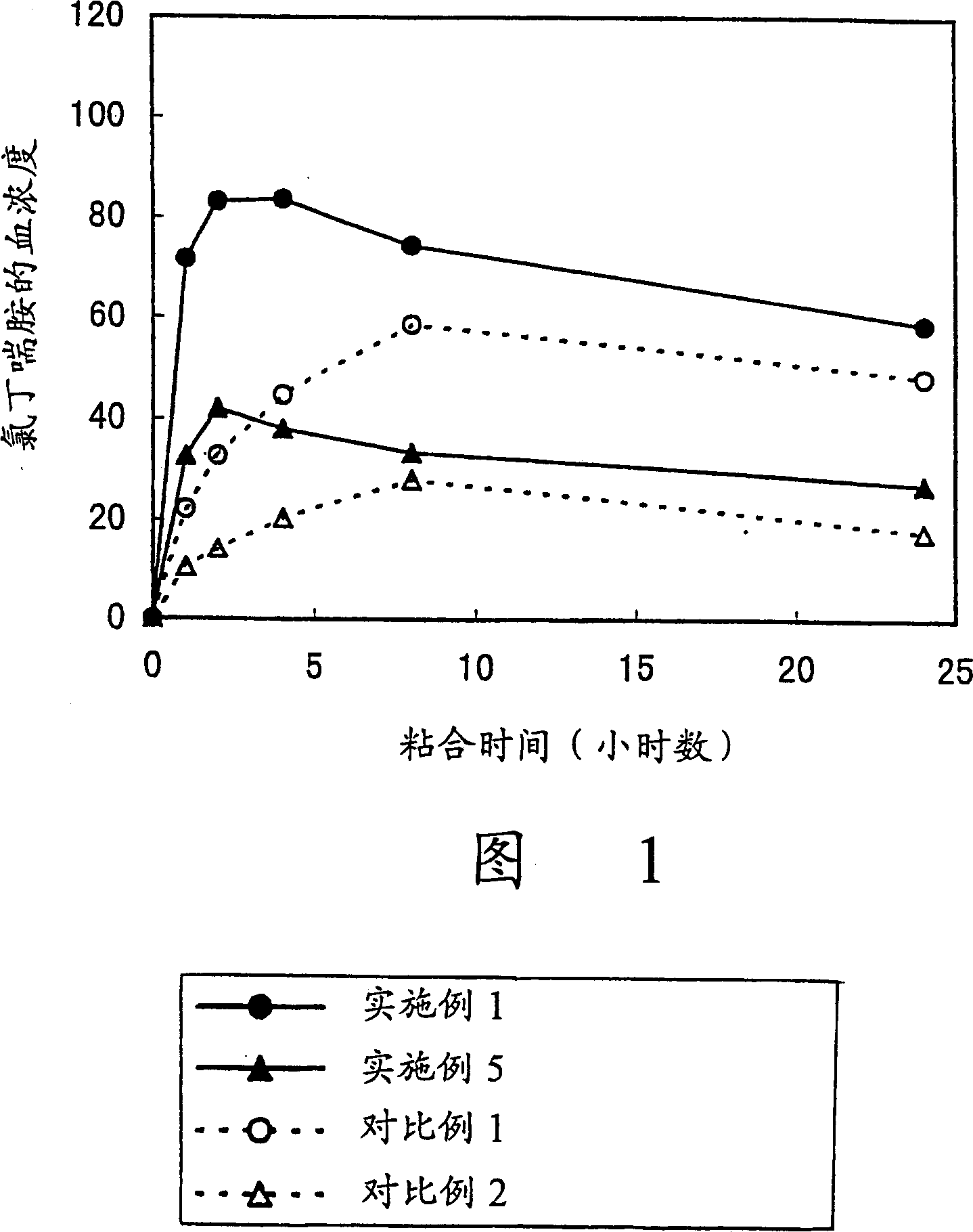
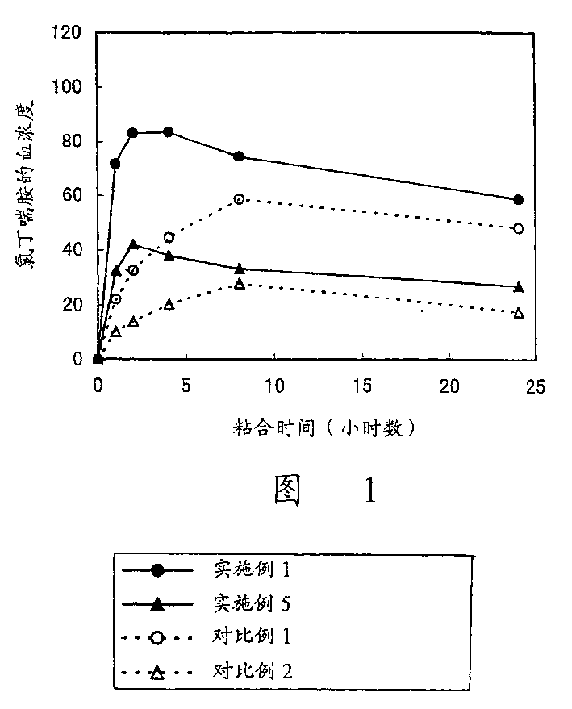
Image
Examples
Embodiment 1
[0051] Polymerization of 2-ethylhexyl acrylate (50 parts), 2-methoxyethyl acrylate (25 parts) and vinyl acetate (25 parts) in ethyl acetate under an inert atmosphere produces a An acrylic adhesive solution. Chlorbuterol was added to the solution so that its content in the plaster layer was 10%, and the mixture was thoroughly stirred. The solution was cast on a pad so that the thickness after drying became 40 µm, and dried to produce a plaster layer. The plaster layer was bonded to a support (12 µm thick polyester film) to obtain a transdermal preparation of the present invention.
Embodiment 2
[0053] In the acrylic adhesive obtained in embodiment 1, add chlorbutazone and polyoxyethylene octyl phenyl ether (the addition mole number of oxyethylene is 3, OP-3, by NIKKO Chemicals CO.LTD. Production), so that their content in the plaster layer is 10%. The mixture was well stirred and in the same manner as in Example 1, a transdermal preparation of the present invention was obtained.
Embodiment 3
[0055] An acrylic binder solution was produced by polymerizing 2-ethylhexyl acrylate (95 parts) and acrylic acid (5 parts) in ethyl acetate under an inert atmosphere. Chlorbuterol and isopropyl myristate as an additive are added to the solution so that their contents in the plaster layer are divided into 20% and 30%, and the mixture is fully stirred. A polyisocyanate compound (trade name: CORONATEHL, produced by NIPPON POL YURETHANE INDUSTRY CO., LTD.) in a proportion of 0.15% of an acrylic binder was added as a crosslinking agent, and the mixture was thoroughly stirred. The solution was cast on a release liner so that the thickness became 60 µm after drying, and dried to obtain a plaster layer. Then, make the plaster layer and the support on the nonwoven side [polyester nonwoven laminated film (basis weight 12g / m 2 ) and 6 μm thick polyester film] bonded to obtain the transdermal preparation of the present invention.
[0056] To accelerate the crosslinking reaction, the for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com