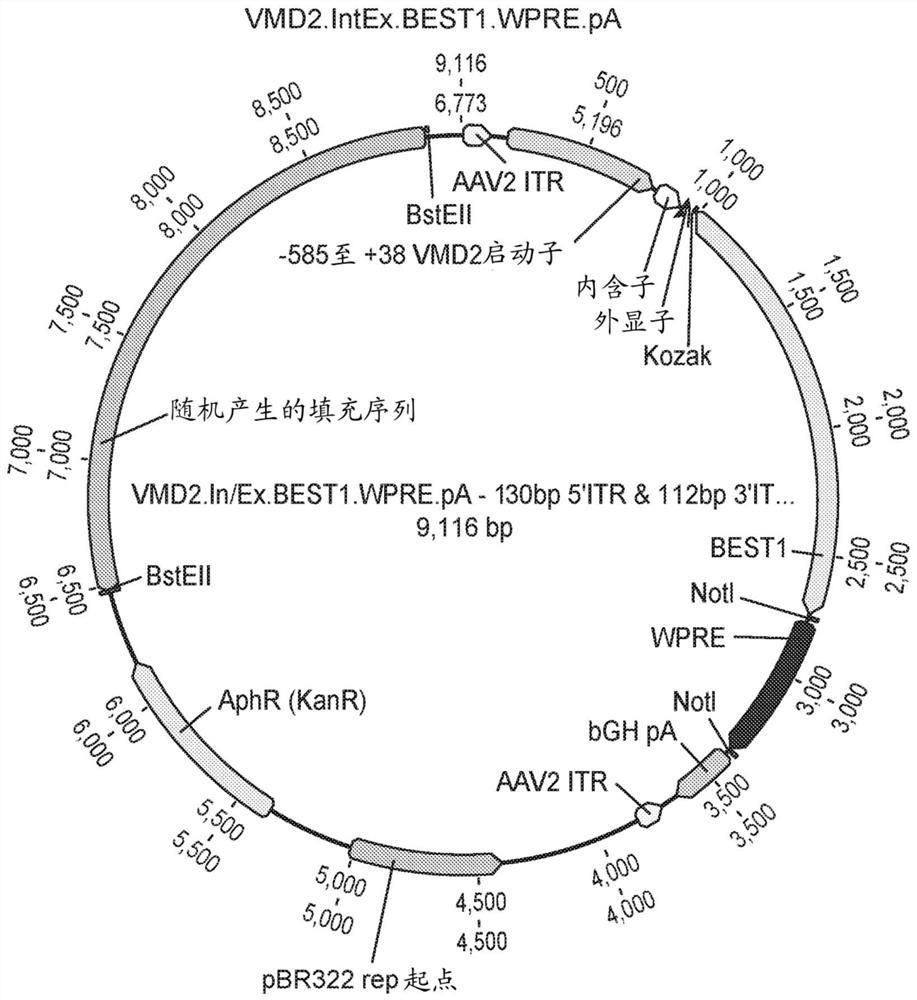
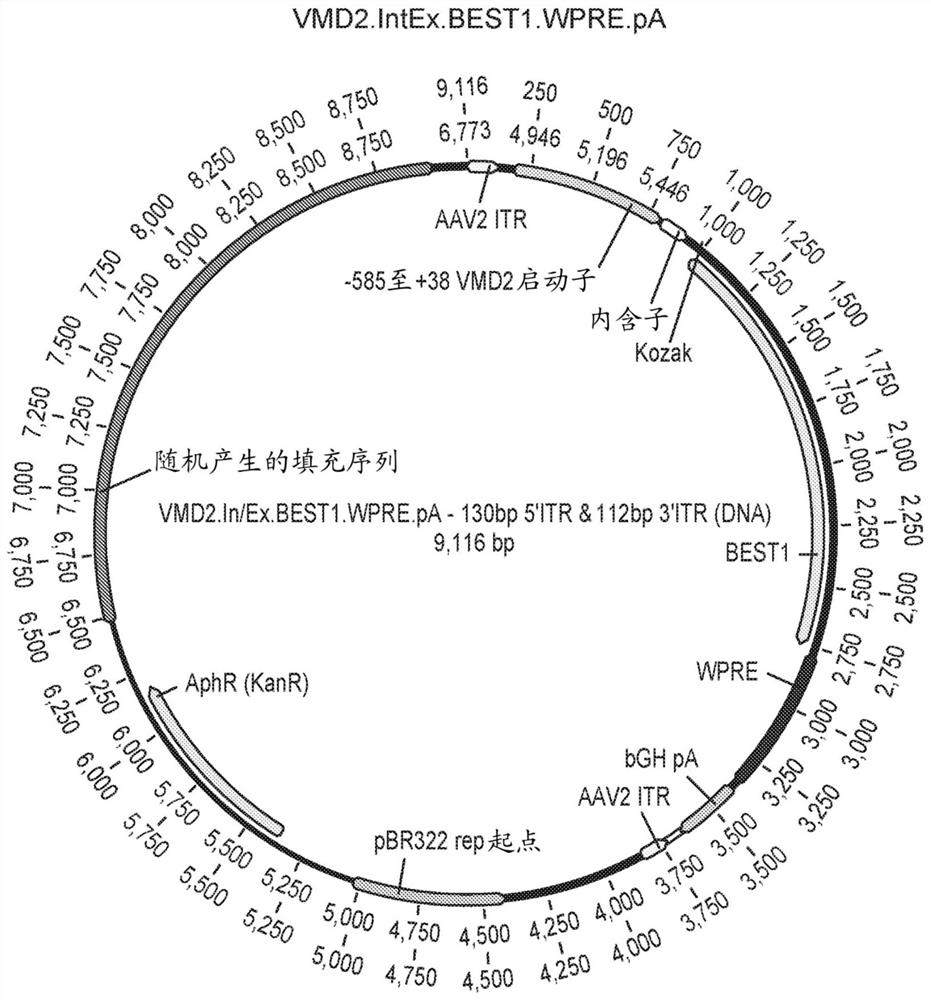
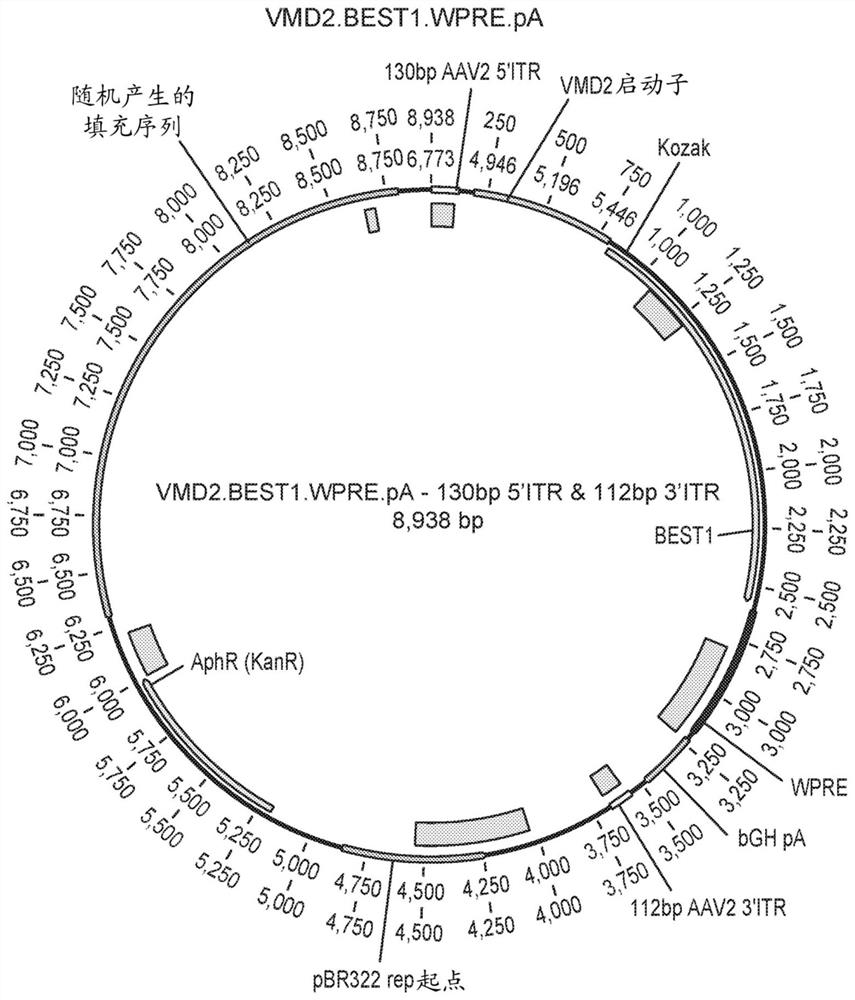
Compositions and methods for treating macular dystrophy
A technology for dystrophic, compositional applications in the field of neurobiology and gene therapy therapy, molecular biology of degenerative eye diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0207] Example 1: Vitelliliform maculopathy-1 protein using the CAG promoter in HEK293 cells
[0208] HEK293 cells were transduced with AAV2 / 2 vectors containing the CAG promoter driving BEST1 expression with WPRE (AAV2 / 2 CAG.BEST1.WPRE.pA, Figure 3) and without WPRE (AAV2 / 2 CAG.BEST1.pA) , and the expression and localization of the vitello-like maculopathy protein-1 protein were examined. In Figure 6, transduced HEK293 cells were stained with Hoechst and anti-human vitelline maculopathy protein-1 (hBEST1 or huBEST1 ) antibodies. Vitellin-1 protein was found throughout the cytosol when compared to non-transduced control cells.
[0209] Vitelligrin-1 expression in HEK293 cells was quantified from Western blot (Fig. 7). exist Figure 7A Among them, sample 1 is the AAV2 / 2 CAG.hBEST1.pA vector; sample 2 is the AAV2 / 2 CAG.hBEST1.WPRE.pA vector; and sample 3 is the negative control. Plasmid-transfected HEK293 cells were used as a positive control. exist Figure 7B Among them, ...
Embodiment 2
[0211] Example 2: Vitelliliform maculopathy-1 protein using the VMD2 promoter in cultured ARPE19 cells
[0212]Properly differentiated ARPE19 is known to have a gene expression profile similar to native retinal pigment epithelial (RPE) cells and can be used as a surrogate for native RPE cells to test gene expression. Differentiated ARPE19 cells were used to test the ability of the VMD2 and CAG promoters to drive BEST1 expression in RPE cells and to test the effect of intron-exon (IntEx) sequences on expression from the VMD2 promoter.
[0213] ARPE19 cells were used Figure 10B Outlined protocol for transfection and assay for BEST1 expression. Arpe19 cells were incubated in 96-well plates at 37 °C and 5% CO 2 Grow in differentiation medium (DMEM with 4.5 g / l of glucose, L-glutamine and 1 mM sodium pyruvate supplemented with 1% fetal bovine serum (FBS)) for 1 to 4 months. Then, the differentiated ARPE19 cells were divided into 3.8×10 plasmids per well. 10 Copy number of pCAG...
Embodiment 3
[0218] Example 3: In vivo pilot study in mice at 4 / 8 weeks
[0219] The ability of the VMD2.BEST1.WPRE and VMD2.IntEx.BEST1.WPRE constructs to drive BEST1 expression was determined in vivo. The protocol for the 4 / 8 week in vivo pilot study is shown in Figure 15 middle. C57BL / 6 mice (6 in each group) were bilaterally injected with prosthetic injection, AAV2 / 2VMD2.BEST1.WPRE or AAV2 / 2VMD2.IntEx.BEST1.WPRE AAV virions. 1μLAAV solution was injected subretinal with a 34-gauge Nanofil needle (WPI#NF34BL-2), 1×10 9 GC / μL / eye. Ocular imaging was performed using optical coherence tomography at weeks 4 and 8 to assess retinal thinning (toxicity), and 3 animals were sacrificed at each time point to determine BEST1 protein expression by immunohistochemistry and Western blotting.
[0220] OCT imaging at weeks 4 and 8 showed that none of the VMD2 constructs exhibited photoreceptor toxicity when compared to prosthetic treatment ( Figure 16 to Figure 18 ).
[0221] Three animals were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com