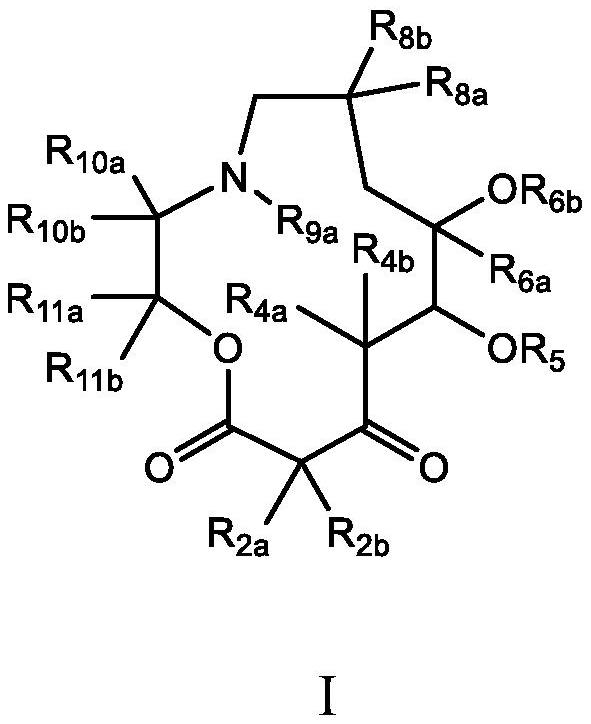
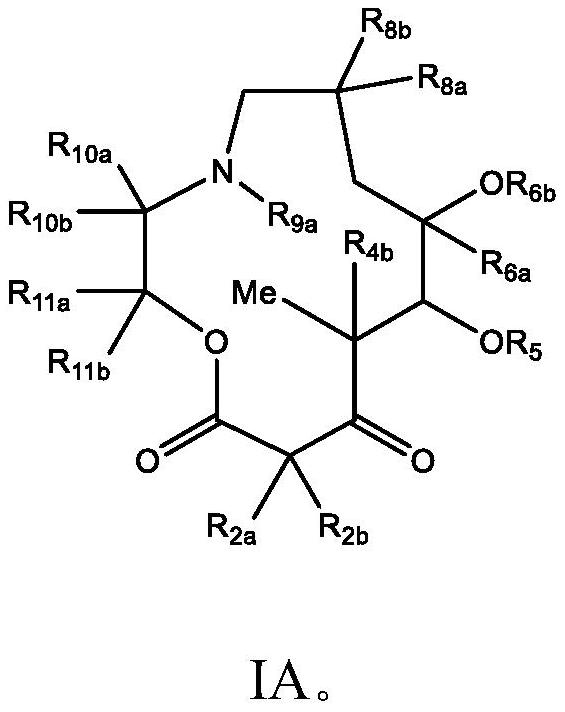
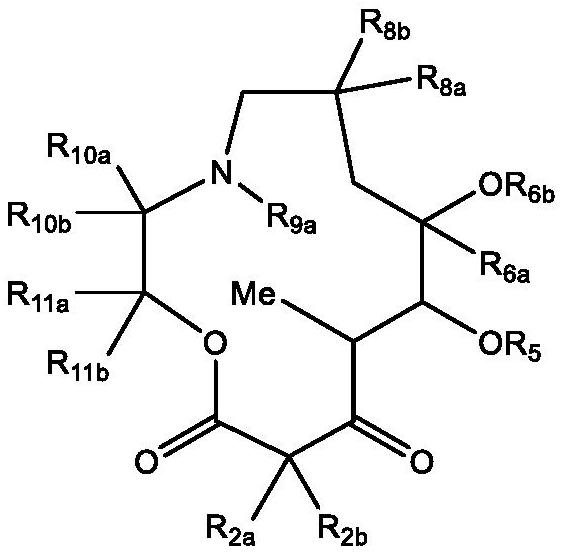
C10-alkylene substituted 13-membered macrolides and uses thereof
A technology of alkylene and alkyl, which is applied in the field of C10-alkylene substituted 13-membered macrolide and its application, and can solve problems such as unresolved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0416] Preparation by coupling and macrolide
[0417] In certain embodiments, as depicted in Scheme 1, macrolides of the present disclosure are obtained by coupling a compound of formula (N-2) (eastern half) with a compound of formula (N-1) (western half) to Provide formula (N-a) non-cyclization macrolide precursor to prepare, wherein R s sugar residue where PG is a protecting group and Indicates a connection point.
[0418] plan 1.
[0419]
[0420] As depicted in Scheme 2, formula (N-a) is cyclized to Obtain formula (I) macrocyclic lactone after deprotection.
[0421] Scenario 2.
[0422]
[0423] Alternatively, as shown in Scheme 3, the macrolide precursor of formula (N-a) is cyclized to provide the macrolide of formula (P) (i.e., the compound of formula (I) where R 9a is hydrogen), which can undergo reductive amination to provide a compound of formula (I), wherein, as otherwise defined for formula I, R 9a Not for H.
[0424] Option 3.
[0425]
[0426...
Embodiment
[0581] In order that the invention described herein may be more fully understood, the following examples are set forth. The synthetic and biological examples described in this application are provided to illustrate the compounds, pharmaceutical compositions and methods provided herein and are not to be construed in any way as limiting the scope thereof.
[0582] Table 1 lists intermediates used to prepare exemplary compounds.
[0583] Table 1.
[0584]
[0585]
[0586]
[0587] Intermediate Scheme 1.
[0588]
[0589] tert-Butyl 4-(((Benzyloxy)carbonyl)-D-threonyl)piperazine-1-carboxylate (IS1-1)
[0590] To a solution of (2R,3S)-2-{[(benzyloxy)carbonyl]amino}-3-hydroxybutanoic acid (3 g, 11.8 mmol) in EtOAc (50 mL) was added DIEA (1.8 mL, 10.3 mmol ) and tert-butylpiperazine (2 g, 10.7 mmol). Add 1-propanephosphonic anhydride by pipette with stirring over 2 minutes (min) (8.63 g of a 50% w / w solution in dichloromethane) and the reaction mixture was stirred ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com