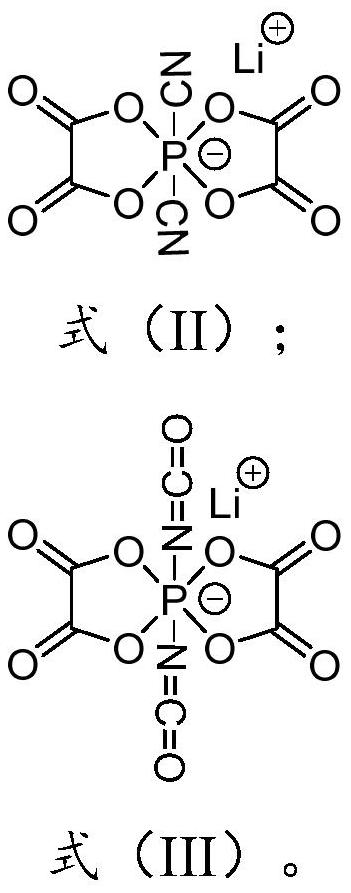
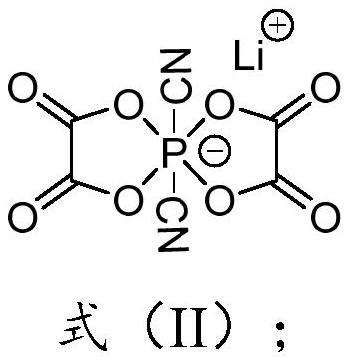
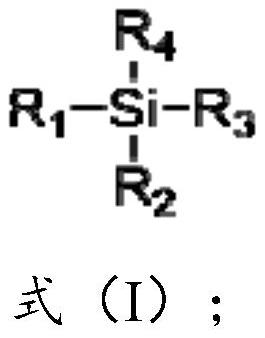Preparation method of lithium difluoro bis (oxalato) phosphate and derivative thereof, electrolyte and secondary battery
A technology of lithium difluorobisoxalate phosphate and lithium bisoxalate phosphate, which is applied in the field of secondary battery materials, lithium difluorobisoxalate phosphate derivatives and its preparation, and the preparation of lithium difluorobisoxalate phosphate, which can solve the problem of chloride ion concentration High free acid and other problems, to achieve the effect of high atom economy, environmental friendliness, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0033] Embodiments of the present invention provide a method for preparing lithium difluoroalkanate, including the steps of:
[0034] S1, providing lithium oxalic acid, lithium hexafluorophosphate, non-aqueous solvent and organic aid;
[0035] S2, in a non-aqueous solvent, lithium oxalic acid, hexafluorophosphate is mixed with an organic aid to obtain a solution of lithium phosphate acid phosphate acid.
[0036] S3, solution of lithium phosphate containing diofluorocyanrate is concentrated to give lithium phosphate phosphate.
[0037]Embodiment of the present invention process for producing lithium difluoro bis (oxalato) phosphate embodiment provided and mixed by the addition of organic additives and oxalic acid and lithium hexafluorophosphate can make otherwise poor solubility in non-aqueous solvent oxalic acid gradually dissolved to give containing titanium liquid lithium bis (oxalato) phosphate fluoride, and then concentrated and dried to give lithium bis (oxalato) phosphate, d...
Example Embodiment
[0110] Example 1
[0111] This embodiment provides a method of preparation of LIDODFP, including the following steps:
[0112] (11) 36 g of oxalic acid and a methane of dimethyl carbonate were added to 500 ml of two bottles, and the solid was not completely dissolved at room temperature, and then 30.4 g of LiPF 6 The 80ml carbonate solution was added to the two flasks, and the stirring system was suspected, and 95 g me 3 SiCl (ME represents methyl) 50 ml carbonate solution is added to the two flask by a dropping funnel, and the dropping acceleration is 1 drop / second, and the gas is released in the dripping process and the insoluble matter is gradually dissolved. The latter insoluble matter is completely dissolved, the solution is a colorless transparent, and stirring at room temperature for 3 h then warmed to 50 ° C, and there is a large amount of HCl gas release, and the temperature is 50 ° C to react 3 h. The HCl gas produced by the reaction was absorbed by sodium hydroxide sa...
Example Embodiment
[0114] Example 2
[0115] This embodiment provides a method of preparation of LIDODFP, including the following steps:
[0116] (21) 36 g of oxalic acid and 100 ml of dimethyl carbonate were added to 500 ml of two bottles, and the solid was stirred at room temperature and was not completely dissolved, and then 30.4 g of LiPF 6 The 80ml carbonate solution was added to the two flasks, and the stirring system was suspected, and 95 g me 3 SiCl's 50 ml carbonate solution was added to the two flask by a dropping funnel, and the drip acceleration was controlled by 1 drop / second. The gas released during the dropwise addition and the insoluble matter gradually dissolved. After all the drops were completed, the insoluble material was completely dissolved. The solution was a colorless transparent, and the stirring was continued for 3 h at room temperature and then warmed to 50 ° C, and there was a large amount of HCl gas released, and the temperature was 50 ° C for 3 h. The HCl gas produced...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap