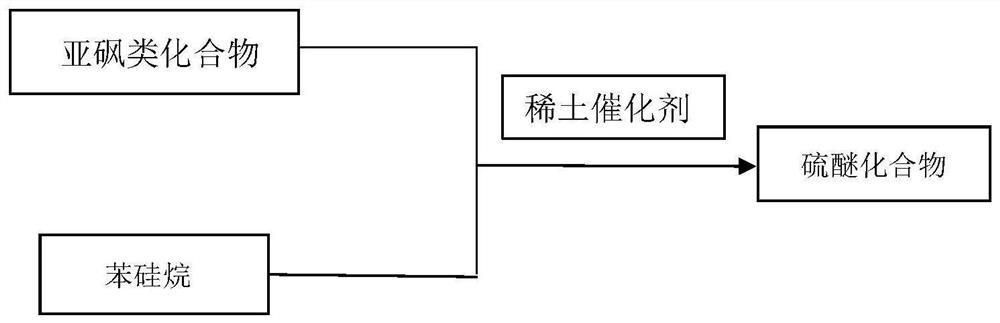
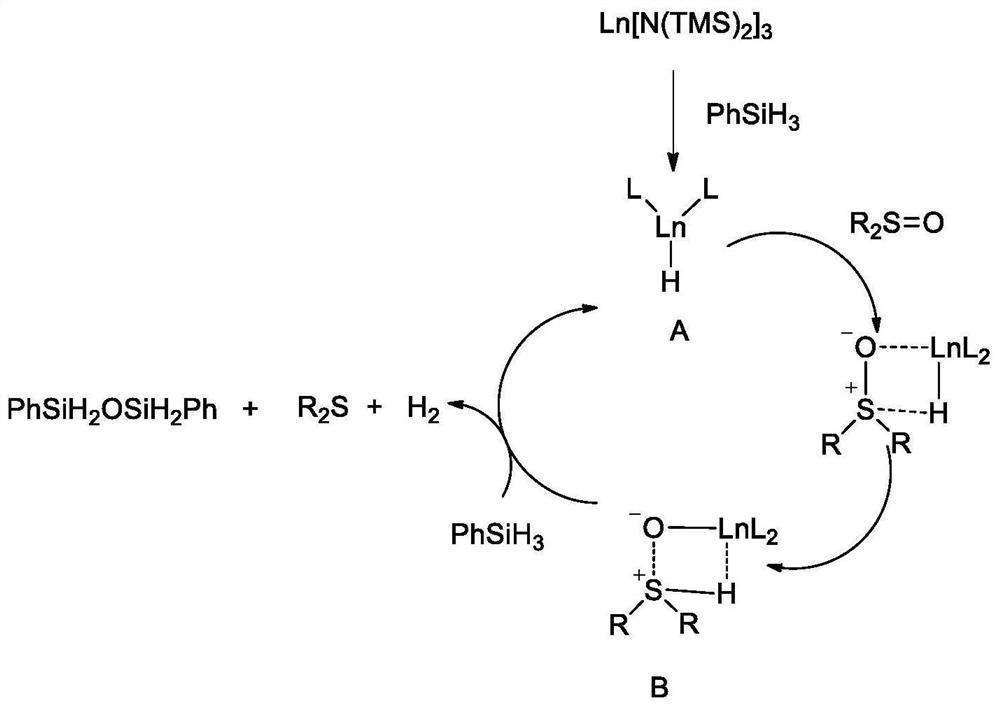
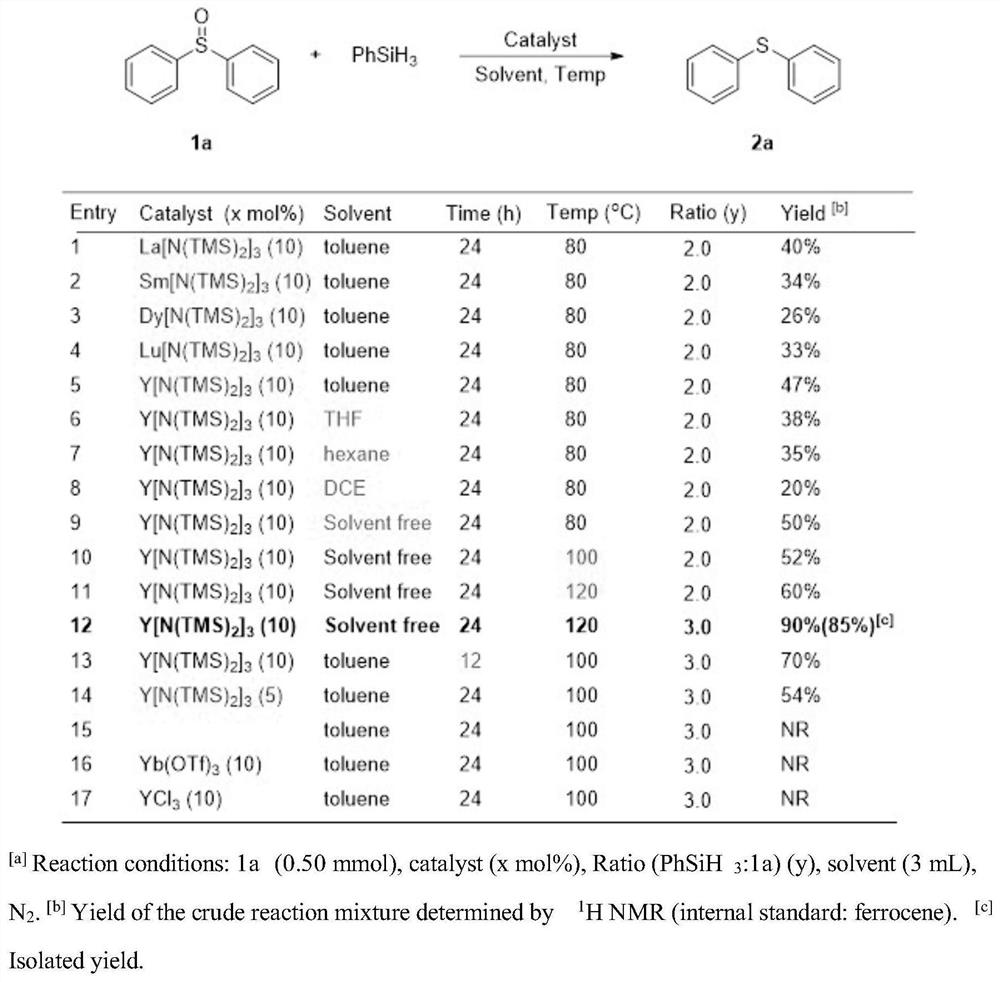
Method for synthesizing thioether compound
A thioether compound and compound technology, applied in the field of thioether compound synthesis, can solve the problems of not easy to obtain reducing agent, low pH value of the reaction system, poor chemoselectivity, etc., to overcome the difficulty of passing through the rare earth catalytic cycle, and to obtain simple , High atomic economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0027] Example 1
[0028] Preparation of diphenyl sulfide, the following structural formula:
[0029]
[0030] Under nitrogen, was added diphenyl sulfoxide starting material (0.3 mmol) and phenyl silane (0.9 mmol), catalyst Y [N (SiMe 3 ) 2 ] 3 (0.03mmol), 120 ℃ reaction 24h, 85% yield of product was isolated.
[0031] The separated product was purified by NMR, and the results are as follows:
[0032] 1 H NMR (CDCL 3 , 500MHz, ppm): δ7.35 (d, J = 7.5Hz, 4H), 7.31 (t, J = 7.5Hz, 4H), 7.25 (t, J = 7.5Hz, 2H).
[0033] 13 C NMR (CDCL 3 , 125MHz, ppm): δ135.93,131.19,129.33,127.18.
Example Embodiment
[0034] Example 2
[0035] 4,4'-xylene sulfide prepared following structural formula:
[0036]
[0037] Under nitrogen was added 4,4'-xylene feed sulfoxide (0.3 mmol) and phenyl silane (0.9 mmol), catalyst Y [N (SiMe 3 ) 2 ] 3 (0.03mmol), for 24h at 120 ℃, product was isolated in 84% yield.
[0038] The separated product was purified by NMR, and the results are as follows:
[0039] 1 H NMR (CDCl3,500MHz, ppm): δ7.25 (d, J = 8.2Hz, 4H), 7.11 (d, J = 8.2Hz, 4H), 2.33 (s, 6H). 13 C NMR (CDCl3,125MHz, ppm) δ137.00,132.78,131.18,130.03,21.20.
Example Embodiment
[0040] Example 3
[0041] Preparation of 4,4'-dichlorodiphenyl sulfide, the following structural formula:
[0042]
[0043] Under nitrogen was added 4,4'-dichlorodiphenyl sulfoxide starting material (0.3 mmol) and phenyl silane (0.9 mmol), catalyst Y [N (SiMe 3 ) 2 ] 3 (0.03mmol), for 48h at 120 ℃, product was isolated in 90% yield.
[0044] The separated product was purified by NMR, and the results are as follows:
[0045] 1 H NMR (CDCL 3 , 400MHz, ppm): δ7.27-7.29 (m, 4H), 7.24-7.26 (m, 4H). 13 C NMR (CDCL 3 , 125MHz, ppm): δ134.08,133.62,132.45,129.64.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap