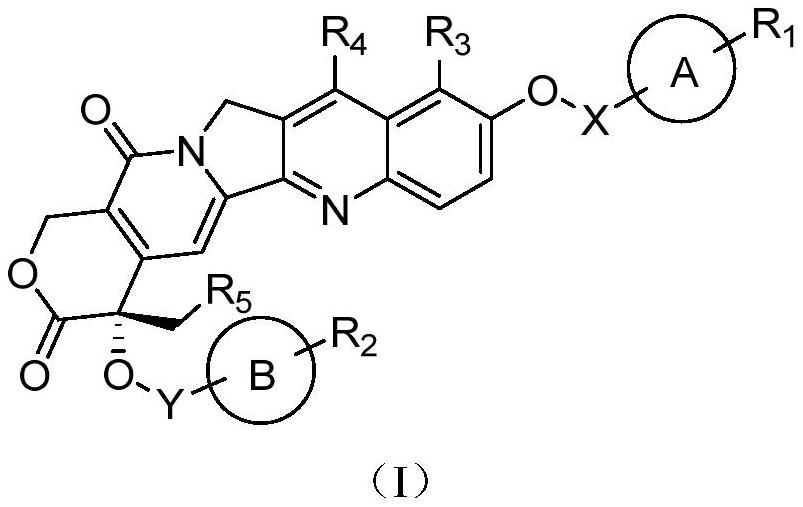
Camptothecin prodrug and application thereof
A technology of camptothecin and prodrug, applied in the field of preparing antitumor drugs, can solve the problems of increasing resistance, reducing therapeutic effect, low activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The camptothecin prodrug 4-ethyl-4-hydroxyl-9-((4-nitrobenzyl)oxy)-1,12-dihydro-14H-pyrano[3',4 ':6,7]indolizine[1,2-b]quinoline-3,14(4H)-dione (No. I-1) is synthesized by one-step reaction, and the reaction formula is as follows:
[0081]
[0082] Dissolve 0.5g (1.37mmol) of camptothecin in 20ml of N,N-dimethylformamide, add 0.38g (2.74mmol) of potassium carbonate, cool to 0°C, and protect with nitrogen. Slowly drop 0.30 g (1.37 mmol) of p-nitrobenzyl bromide dissolved in 5 mL of N,N-dimethylformamide with a syringe. After the addition, it was slowly raised to room temperature and reacted at room temperature for 12 hours. After the reaction, potassium carbonate was removed by filtration, and the filtrate was concentrated to obtain a crude product, which was separated by silica gel column chromatography and eluted with dichloromethane:methanol (20:1) to obtain 0.57 g of white solid (I-1), with a yield of 83.2%.
[0083] NMR characterization of I-1:
[0084] 1 H N...
Embodiment 2
[0087] The camptothecin class prodrug 4,11-diethyl-4-hydroxyl-9-((4-nitrobenzyl)oxy)-1,12-dihydro-14H-pyran[3 ',4': 6,7]indolizine[1,2-b]quinoline-3,14(4H)-dione (No. I-2) is synthesized by reaction, and the reaction formula is as follows:
[0088]
[0089] Dissolve 0.5g (1.27mmol) of hydroxycamptothecin in 20ml of N,N-dimethylformamide, add 0.35g (2.57mmol) of potassium carbonate, cool to 0°C, and protect with nitrogen. 0.28 g (1.27 mmol) of p-nitrobenzyl bromide dissolved in 5 mL of N,N-dimethylformamide was slowly dropped from a syringe. After the addition was completed, the temperature was slowly raised to 80° C., and the mixture was refluxed for 12 hours. After the reaction, potassium carbonate was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a crude product, which was separated by silica gel column chromatography and eluted with dichloromethane: methanol (2:1) to obtain 0.51 g of white solid (I-2), with a yield of 75.9 %....
Embodiment 3
[0094] Study on the degradation of camptothecin prodrugs mediated by tumor microenvironment (nitroreductase): Dissolve I-1 or I-2 in 15mL of 10mmol / L Tris buffer, add appropriate amount of nitroreductase and smoke Amide adenine dinucleotide or nicotinamide adenine dinucleotide phosphate, diluted to 20mL with 10mmol / L Tris buffer, measured by HPLC 100s, 200s, 300s, 400s, 500s after adding nitroreductase buffer , 600s... After the concentration of camptothecin and I-1 or hydroxycamptothecin and I-2, to determine whether the target compound can be degraded in the presence of nitroreductase. The results showed that compounds I-1 and I-2 could be degraded rapidly under the condition of nitroreductase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com