Combined boron cage ionic compound fast ion conductor material and preparation method thereof
A technology of ionic compounds and ionic conductors, which can be used in the manufacture of final products, electrochemical generators, sustainable manufacturing/processing, etc. It can solve the problems of easily damaged anode materials, flammability, and explosion, and achieve good thermal/chemical stability. properties, excellent room temperature ionic conductivity, and the effect of high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1, Na 2 B 20 h 18 Fast ion conductor preparation
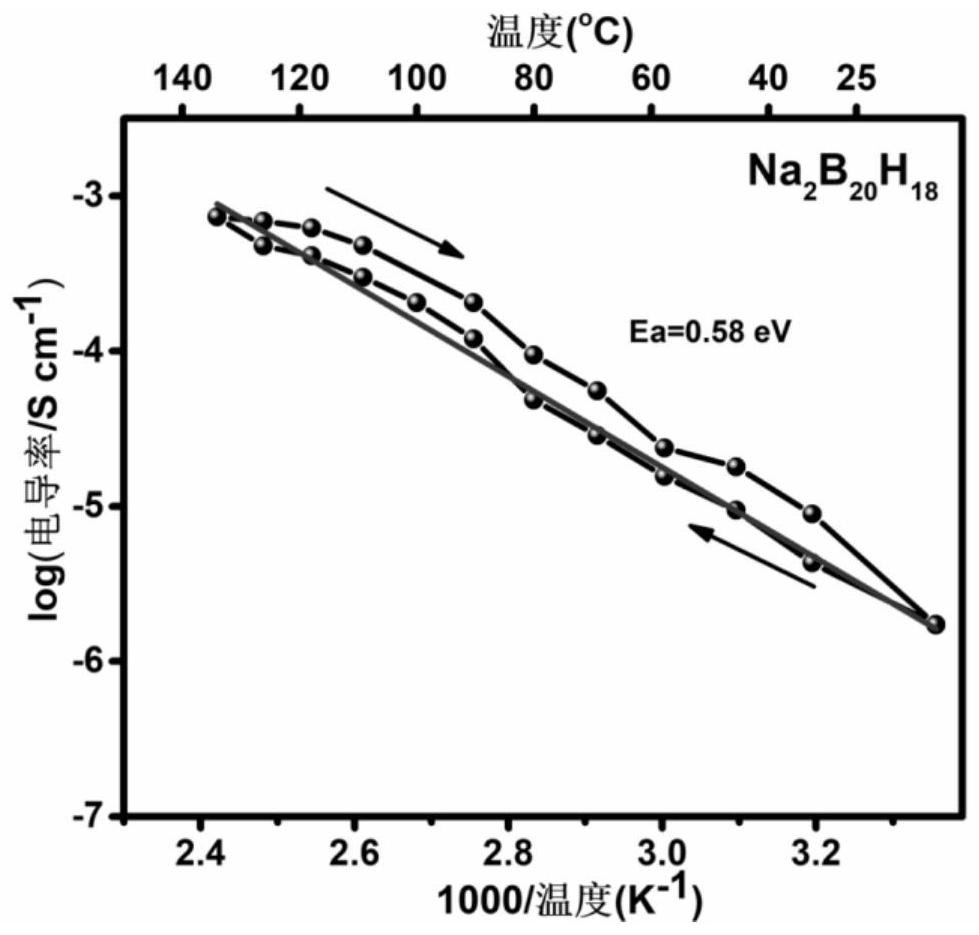
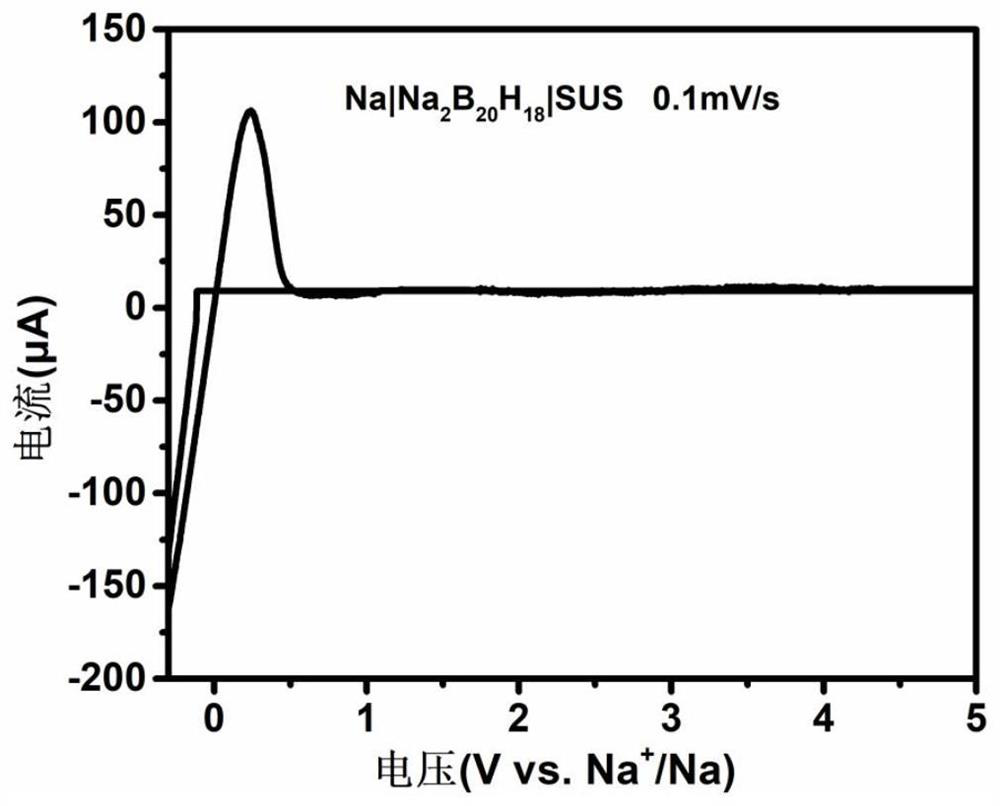
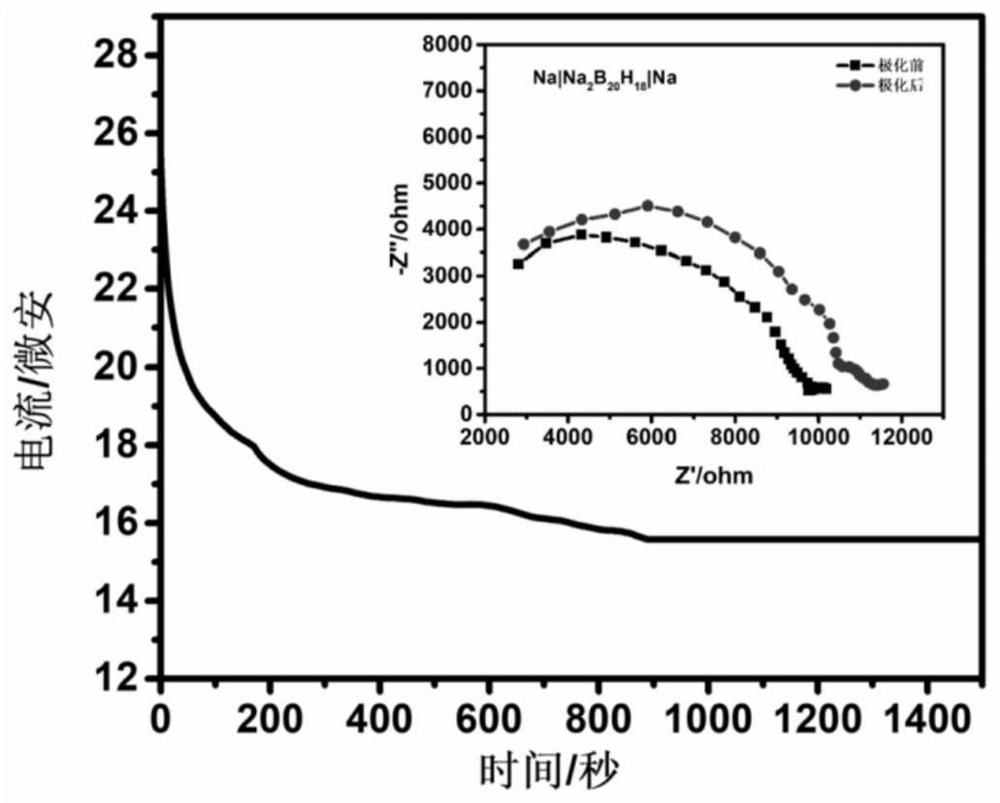
[0026] Take dry (Et 4 N) 2 B 20 h 18 Dissolved in acetonitrile / water solvent, after passing through strongly acidic cationic resin, (H 3 O) 2 B 20 h 18 solution, and then reacted with aqueous sodium hydroxide solution to obtain Na 2 B20 h 18 solution, after drying by rotary evaporation, the product was heated in vacuum at 150 °C for 3.0 hours to obtain Na 2 B 20 h 18 . The conductivity of the fast ion conductor was tested with an electrochemical workstation (CHI760E, Shanghai) as the detection equipment, using a three-electrode system, and the solid fast ion conductor Na 2 B 20 h 18 Press the tablet to make a test sheet with a certain thickness and area; assemble it from bottom to top according to the stainless steel substrate, fast ion conductor test piece, and stainless steel substrate, and conduct AC impedance (AC impedance) tests at different temperatures, and according to the formula : σ =...
Embodiment 2
[0027] Example 2, Li 2 B 20 h 18 Fast ion conductor preparation
[0028] The dried (Et 4 N) 2 B 20 h 18 Dissolved in solvents such as acetonitrile / water, after passing through a strongly acidic cationic resin, (H 3 O) 2 B 20 h 18 , and then react with lithium hydroxide solution to obtain Li 2 B 20 h 18 Solution, after drying by rotary evaporation, the product was heated at 150°C for 3.0 hours to obtain the fast ion conductor Li 2 B 20 h 18 . At different temperatures, conduct AC impedance tests and calculate the ionic conductivity of the material at different temperatures according to the formula, the results are as follows Figure 4 shown. The calculation results show that at room temperature Li 2 B 20 h 18 The ionic conductivity is 1.1×10 -6 S / cm.
Embodiment 3
[0029] Example 3, Na 2 B 20 h 18 / Na 2 B 12 h 12 composite fast ion conductor
[0030] the dried Na 2 B 20 h 18 and Na 2 B 12 h 12 Mix them in molar ratios of 1:1, 1:2, 1:3, 1:4, 1:5 and 1:6 respectively, add them to the ball mill jar respectively, and ball mill 3.0 at the speed of 400 rpm under the condition of isolating the air. hours, get Na 2 B 20 h 18 / Na 2 B 12 h 12 Composite fast ion conductor. At different temperatures, AC impedance tests were carried out and the ionic conductivity of each material at different temperatures was calculated according to the formula. The results are as follows Figure 5 shown. The calculation results show that at room temperature Na 2 B 20 h 18 -4Na 2 B 12 h 12 The highest conductivity is 2.8×10 -4 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com