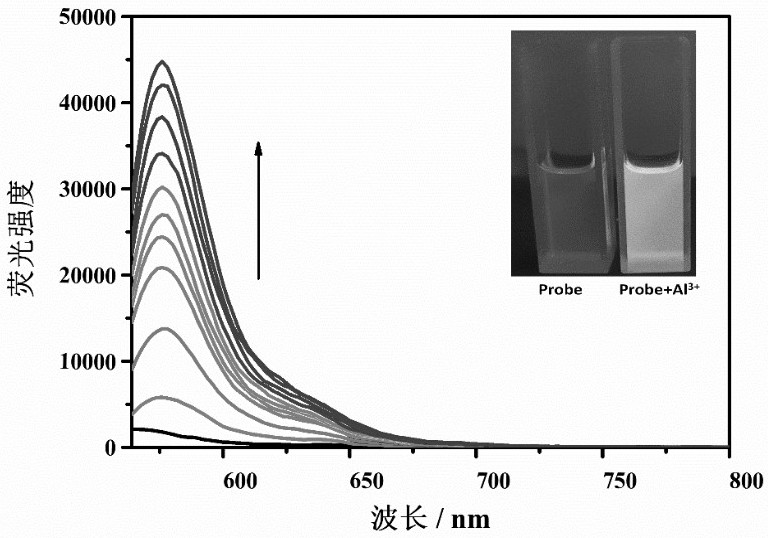
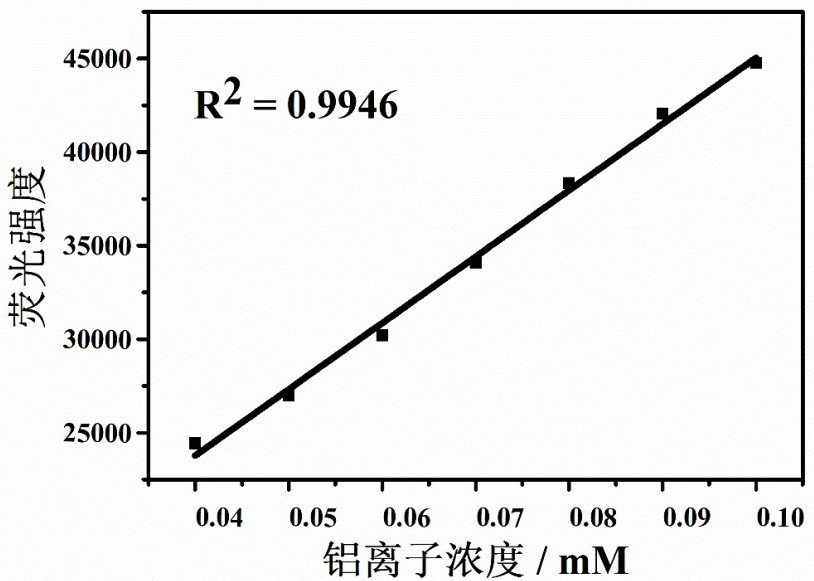
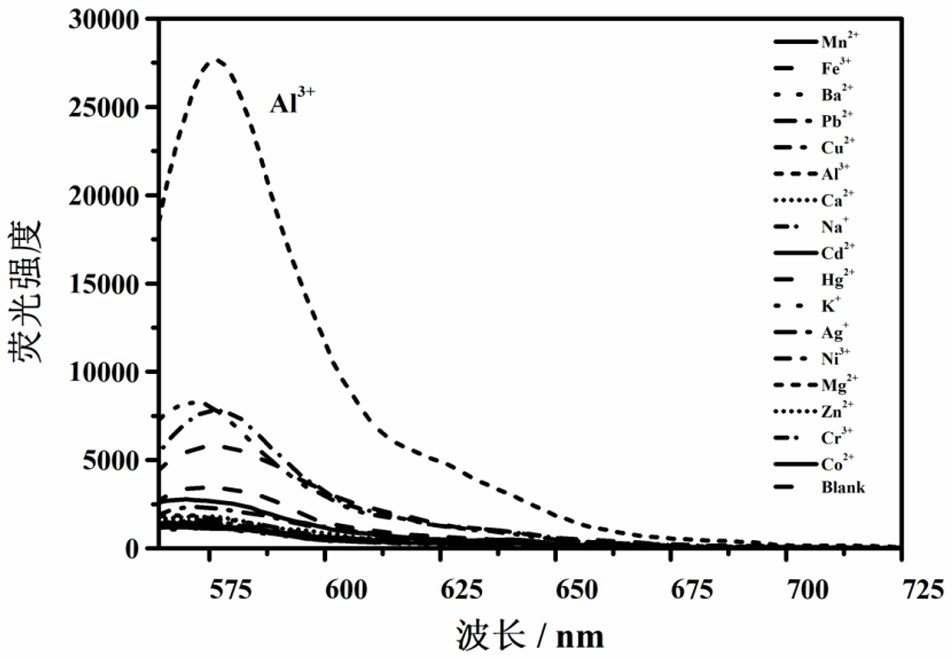
Lysosome targeting rhodamine B hydrazide fluorescent probe as well as preparation method and application thereof
A fluorescent probe and targeting technology, applied in the field of probes, can solve the problem of less fluorescent probes, and achieve the effects of simple synthesis method, high selectivity, and obvious changes in fluorescence intensity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Rhodamine B Hydrazide
[0039] In a 250 mL round bottom flask, 3.832 g of rhodamine B (8 mmol), 8 mL of hydrazine hydrate (132 mmol) and 100 mL of anhydrous methanol were added, and the mixture was stirred. The mixture was heated to reflux, maintained at 65°C for 3 hours, cooled to room temperature of 25°C, and the methanol solvent was removed by rotary evaporation under reduced pressure. The residue was dissolved in 30 mL of hydrochloric acid (1 M), and NaOH solution (1 M) adjust the pH to 9, generate a large amount of light pink solids, suction filter, wash the filter cake with ice water several times, and vacuum dry to obtain 3.3642 g of light pink powdery solids with a yield of 92.1%. m.p. 215-217ºC.
[0040] Synthesis and characterization of lysosome-targeted rhodamine B hydrazide fluorescent probe RM
[0041] In a 50 mL round-bottom flask, 0.23 g (0.50 mmol) of rhodamine B hydrazide, 0.14 g (0.50 mmol) of 4-morpholinyl benzaldehyde, and 10 mL of...
Embodiment 2
[0044] Synthesis of Rhodamine B Hydrazide
[0045] In a 250 mL round bottom flask, 3.832 g of rhodamine B (8 mmol), 8 mL of hydrazine hydrate (132 mmol) and 100 mL of anhydrous methanol were added, and the mixture was stirred. The mixture was heated to reflux and kept at 50 °C for 4 hours. After cooling to 20 °C, the methanol solvent was removed by rotary evaporation under reduced pressure. The residue was dissolved in 30 mL of hydrochloric acid (1 M) and adjusted with NaOH solution (1 M). pH=9.0, a large amount of light pink solids were formed, suction filtration, the filter cake was washed several times with ice water, and dried in vacuo to obtain 3.3642 g of light pink powdery solids with a yield of 92.1%. m.p. 215-217ºC.
[0046] Synthesis and characterization of lysosome-targeted rhodamine B hydrazide fluorescent probe RM
[0047] In a 50 mL round-bottom flask, 0.23 g (0.50 mmol) of rhodamine B hydrazide, 0.14 g (0.50 mmol) of 4-morpholinyl benzaldehyde, and 10 mL o...
Embodiment 3
[0049] Synthesis of Rhodamine B Hydrazide
[0050] In a 250 mL round bottom flask, 3.832 g of rhodamine B (8 mmol), 8 mL of hydrazine hydrate (132 mmol) and 100 mL of anhydrous methanol were added, and the mixture was stirred. The mixture was heated to reflux, kept at 70 °C for 2 hours, cooled to 30 °C, the methanol solvent was removed by rotary evaporation under reduced pressure, the residue was dissolved in 30 mL of hydrochloric acid (1 M), and adjusted with NaOH solution (1 M) pH=9.0, a large amount of light pink solids were formed, suction filtration, the filter cake was washed several times with ice water, and dried in vacuo to obtain 3.3642 g of light pink powdery solids with a yield of 92.1%. m.p. 215-217ºC.
[0051] Synthesis and characterization of lysosome-targeted rhodamine B hydrazide fluorescent probe RM
[0052] In a 50 mL round-bottom flask, 0.23 g (0.50 mmol) of rhodamine B hydrazide, 0.14 g (0.50 mmol) of 4-morpholinyl benzaldehyde, and 10 mL of absolute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com