Preparation method of (S)-1-(3-bromo-2-methoxyphenyl) ethyl-1-ol
A technology of methoxyphenyl and methoxyacetophenone, which is applied in the field of preparation of chiral pharmaceutical intermediates, can solve problems such as unfavorable scale-up production, low overall yield, material waste, etc., and achieves short route and process yield. The effect of high rate and easy industrial amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
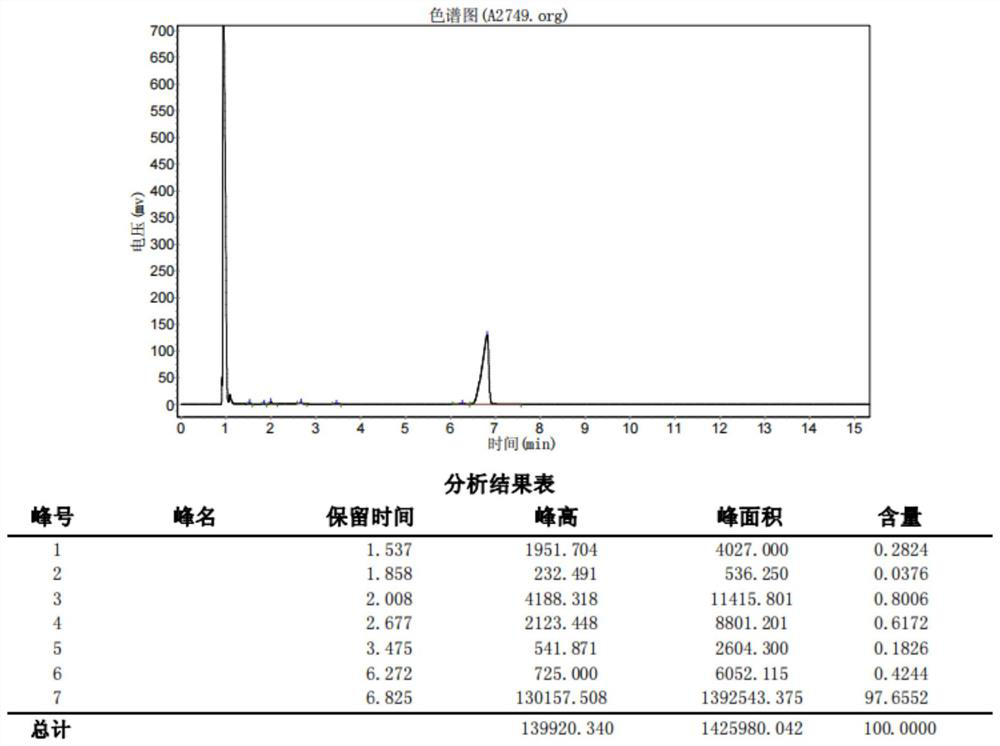
[0082] like figure 1 As shown, under nitrogen protection, compound 9 (25.0g, 100mmol), methyl iodide (28.4g, 200mmol) and potassium carbonate (20.7g, 150mmol) and 200ml of acetone were added to a 500ml four-necked flask, heated at 42°C The reaction was kept under reflux for 18 hours, then cooled to room temperature, and the solvent was removed under reduced pressure (15 mmHg) at 35°C. After removing acetone, the resulting mixture was poured into 120 ml of water, extracted with 50 ml of dichloromethane, and the organic phase was washed with 100 ml of saturated brine. The phase was dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure (15mmHg) at 35°C, and the 92°C-94°C fraction was collected by high vacuum (2mmHg) distillation to obtain intermediate 7 (25.1g), purity (GC) 97.65%, yield 95.08%.
Embodiment 2
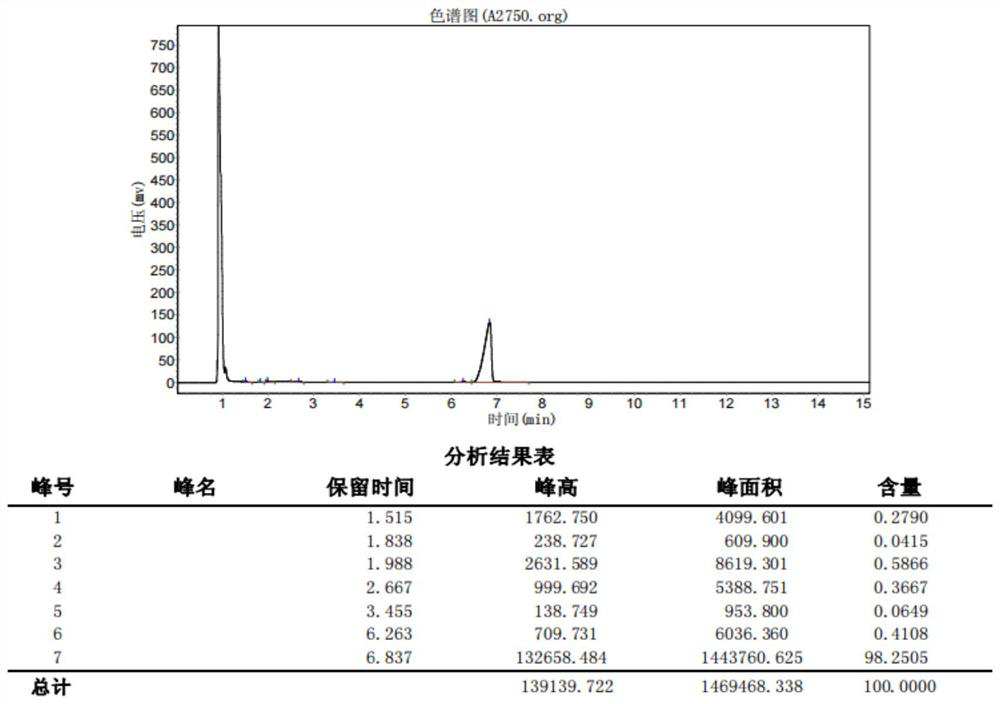
[0084] like figure 2 As shown, under nitrogen protection, compound 9 (25.0g, 100mmol), dimethyl sulfate (18.9g, 150mmol) and potassium carbonate (41.4g, 300mmol) and 300ml of acetone were added to a 500ml four-necked flask, heated The reaction was maintained at 58°C under reflux for 18 hours, then cooled to room temperature, and the solvent was removed at 35°C under reduced pressure (15 mmHg). After removing the acetone, the resulting mixture was poured into 120 ml of water, extracted with 50 ml of dichloromethane, and the organic phase was washed with 100 ml of saturated brine. , the organic phase was dried with anhydrous sodium sulfate, the solvent was removed under reduced pressure (15mmHg) at 35°C, and the 92°C-94°C fraction was collected by high vacuum (2mmHg) distillation to obtain Intermediate 7 (23.5g), purity (GC) 98.25%, Yield 89.02%.
[0085] Preparation of 2-methoxyacetophenone
Embodiment 3
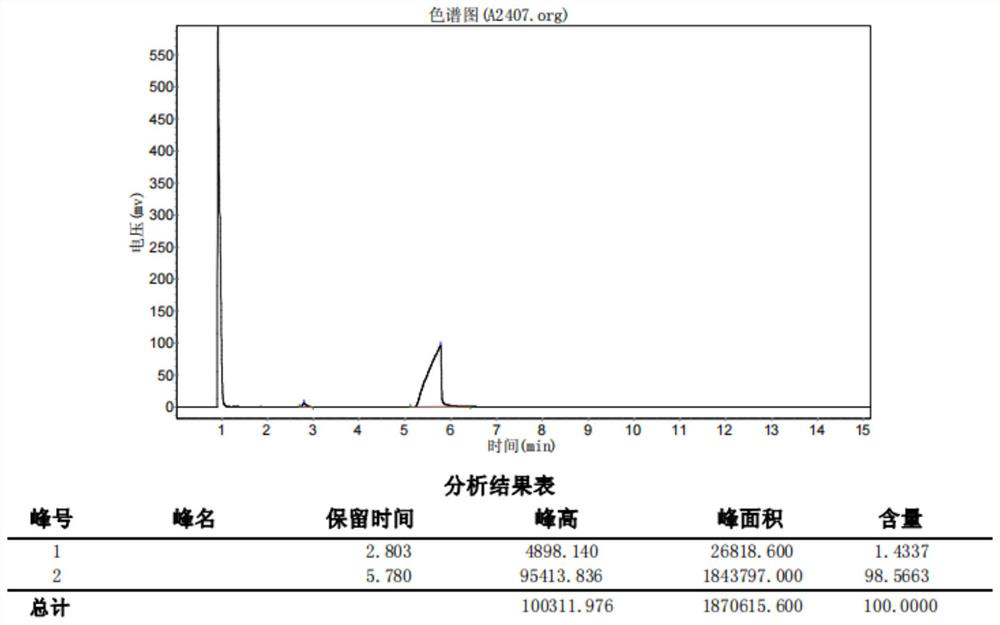
[0087] like image 3 As shown, under nitrogen protection, tetrahydrofuran (240ml), intermediate 7 (26.4g, 100mmol) and the tetrahydrofuran solution (75ml, 150mmol) of isopropylmagnesium chloride were added to a 500ml four-necked flask, maintaining a temperature of 0-5 ℃ , be heated to 25 ℃ after stirring for 4 hours, continue to stir for 2 hours, then add N-methoxy-N-methylacetamide (15.5g, 150mmol) dropwise to the reaction system, keep the temperature not higher than 25 ℃, dropwise After the addition was completed, the reaction was continued for 5 hours, the reaction solution was poured into 5L of ammonium chloride aqueous solution, the layers were separated, the aqueous phase was extracted with 500ml of ethyl acetate, the organic phases were combined, the organic phase was washed with 100ml of saturated brine, and washed with anhydrous sodium sulfate. Dry, remove the solvent under reduced pressure (15mmHg) at 40°C, and collect 95°C-98°C fractions by high vacuum (2mmHg) disti...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap