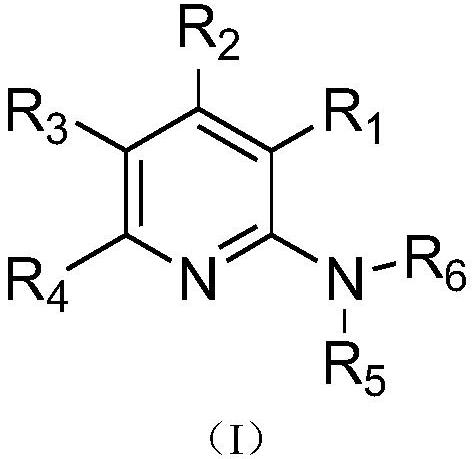
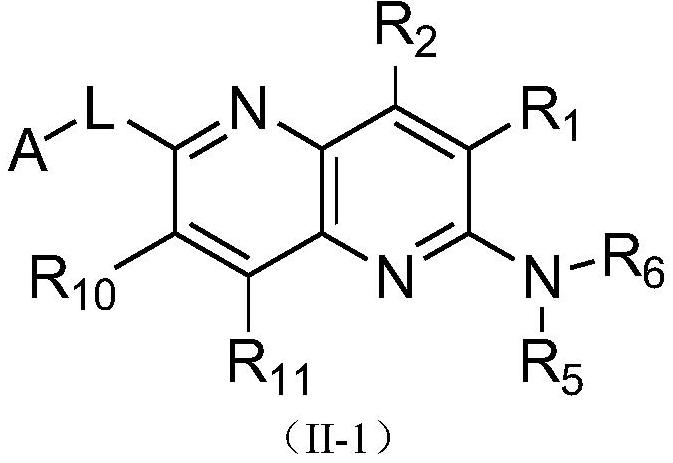
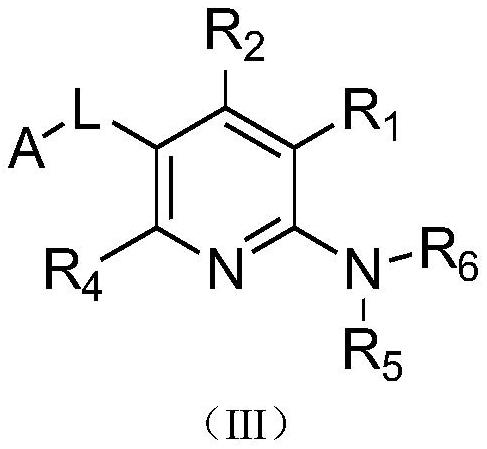
Pyridine-2-amine derivative as well as pharmaceutical composition and application thereof
A technology of amine derivatives and derivatives, applied in the field of pyridin-2-amine derivatives and their pharmaceutical compositions, can solve the problems of unresearched therapeutic prospects and few TLR8 agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1: the preparation method of compound 3, the step comprises:
[0106] (1) Add methyl 3-amino-5-bromopyrimidinecarboxylate (2.3 g, 10.0 mmol) into a 100 mL round-bottomed flask, add 30 mL of tetrahydrofuran to dissolve, cool to 0°C, and slowly add lithium tetrahydroaluminum (760.0 mg) in batches , 20.0 mmol), continued to stir at 0 °C for 1.5 hours, after the reaction was completed, 0.76 mL of water, 0.76 mL of 10% NaOH aqueous solution, and 2.28 mL of water were added to the reaction solution to quench the reaction. After ultrasonication for 10 min, the filter residue was washed with acetone. The filtrate was concentrated under reduced pressure and dried in vacuo to obtain a pale yellow solid 1 (yield=58.0%), the reaction formula was:
[0107]
[0108] (2) Dissolve compound 1 (1.1 g, 5.0 mmol) in 20 mL of dichloromethane, add manganese dioxide (0.97 g, 10.0 mmol), react at room temperature overnight, after the reaction finishes, filter the reaction solutio...
Embodiment 2
[0113] Embodiment 2: the preparation method of compound 6, the step comprises:
[0114] (1) Under nitrogen protection, compound 3 (588.0 mg, 2.0 mmol) obtained in Example 1 was dissolved in 5 mL of dry tetrahydrofuran, and methyl p-formate benzyl bromide (687.0 mg, 3.0 mmol), zinc powder ( 392.4mg, 6.0mmol) and Ni (PPh 3 ) 2 Cl 2 (170.1 mg, 0.26 mmol), the reaction solution was stirred at room temperature for 8 hours, after the reaction was completed, concentrated under reduced pressure, purified by column chromatography (PE:EA=1:1) to obtain compound 4, and the reaction formula was:
[0115]
[0116] (2) Dissolve compound 4 (544.8 mg, 1.5 mmol) in tetrahydrofuran (10 mL), cool to 0 °C, slowly add lithium tetrahydroaluminum (114.0 mg, 3.0 mmol) in batches, continue stirring at 0 °C for 2 hours, After the reaction, 114 μL of water, 114 μL of 10% NaOH aqueous solution, and 342 μL of water were sequentially added to the reaction solution to quench the reaction, sonicated fo...
Embodiment 3
[0120] Embodiment 3: the preparation method of compound N1, the step comprises:
[0121] Using compound 6 prepared in Example 2 as a raw material, compound 6 (88.3 mg, 0.25 mmol) was dissolved in 5 mL of dry methanol, followed by adding dimethylamine (0.375 mL, 2 M in methanol), potassium carbonate (69.1 mg, 0.5 mmol), stirred at 45 ° C for 4 hours, after the reaction was completed, the reaction solution was filtered, the filtrate was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and silica gel column chromatography obtained pale yellow solid N1, the reaction formula is:
[0122]
[0123] 1 H NMR(400MHz, Chloroform-d)δ7.95(d,J=8.6Hz,1H),7.84(d,J=8.6Hz,1H),7.27(m,4H),5.00(s,2H),4.29 (s, 2H), 3.47(s, 2H), 2.61(t, J=7.7Hz, 2H), 2.29(s, 6H), 1.80(p, J=7.4Hz, 2H), 1.44(tq, J= 13.7,7.8,6.0Hz,4H),0.95(t,J=6.8Hz,3H). 13 C NMR (101MHz, Chloroform-d) δ157.27, 156.02, 140.58, 140.25, 138.64, 135.99, 133.56, 129.54, 129.10, 127.41, 124.01, 65.77, 45.04, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com