Polyasparagine derivative containing high-activity hydrosy radical
A technology for polyasparagine and derivatives, which is applied in the field of polyasparagine derivatives, can solve the problems of unfavorable polar medicinal chemical bonding, unfavorable chemically bound medicines, small number of active hydroxyl groups, etc., and achieves that the preparation method is simple and feasible, Widespread application prospects and the effect of large drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
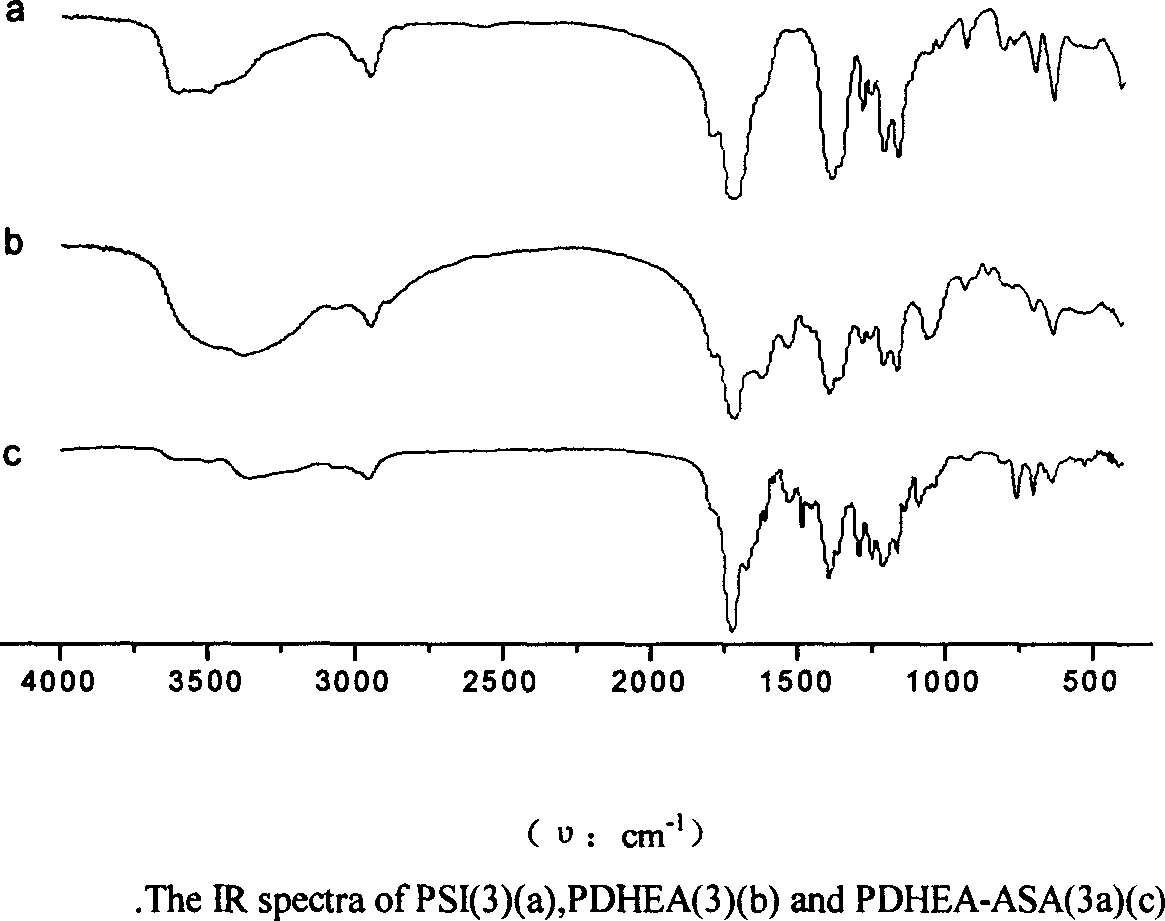
[0017] The present invention will be described in further detail below in conjunction with the accompanying drawings.
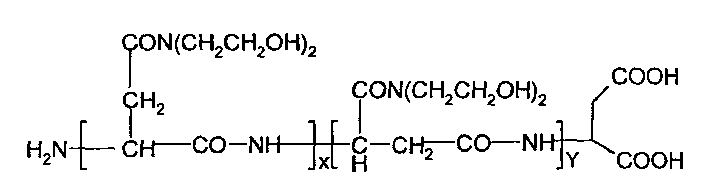
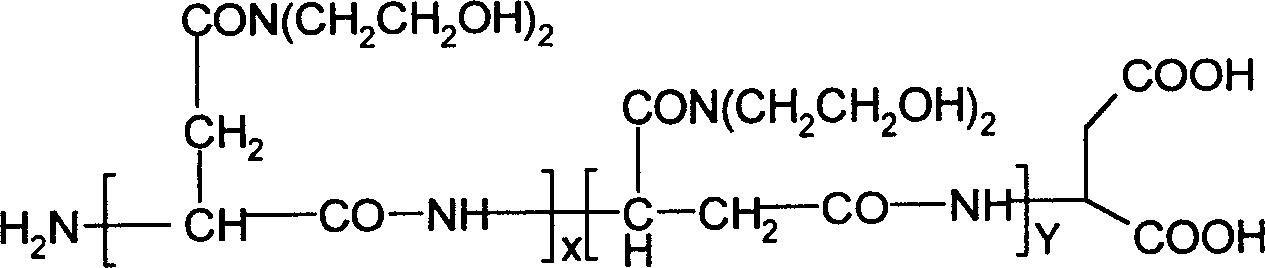
[0018] According to the present invention, a polyasparagine derivative rich in active hydroxyl groups is prepared. Its preparation method is as follows: firstly weigh 200 grams of aspartic acid, add 10 grams of catalyst concentrated phosphoric acid, and carry out polycondensation reaction at 180 ° C. React for 10 hours, gradually reduce the pressure to 1×10 4 Pa, polysuccinimide (code name: PSI) can be obtained with a yield of 95%; then 50 grams of PSI are dissolved in 250ml N,N-dimethylformamide (code name: DMF), cooled to 0°C At this temperature, 120 milliliters of diethanolamine was added dropwise, reacted at room temperature for 4 hours, the mixture was added dropwise to 1500 milliliters of n-butanol and stirred continuously, filtered after standing for 1 hour, washed with acetone until the pH value of the filtrate was neutral , vacuum-dried at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com