Acraldehyde recovering method
A recovery method and acrolein technology, applied in the separation/purification of carbonyl compounds, etc., can solve the problems of affecting the life of the hydrogenation catalyst, difficult to reduce the content of acrolein, and unsuitable for separation of acrolein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
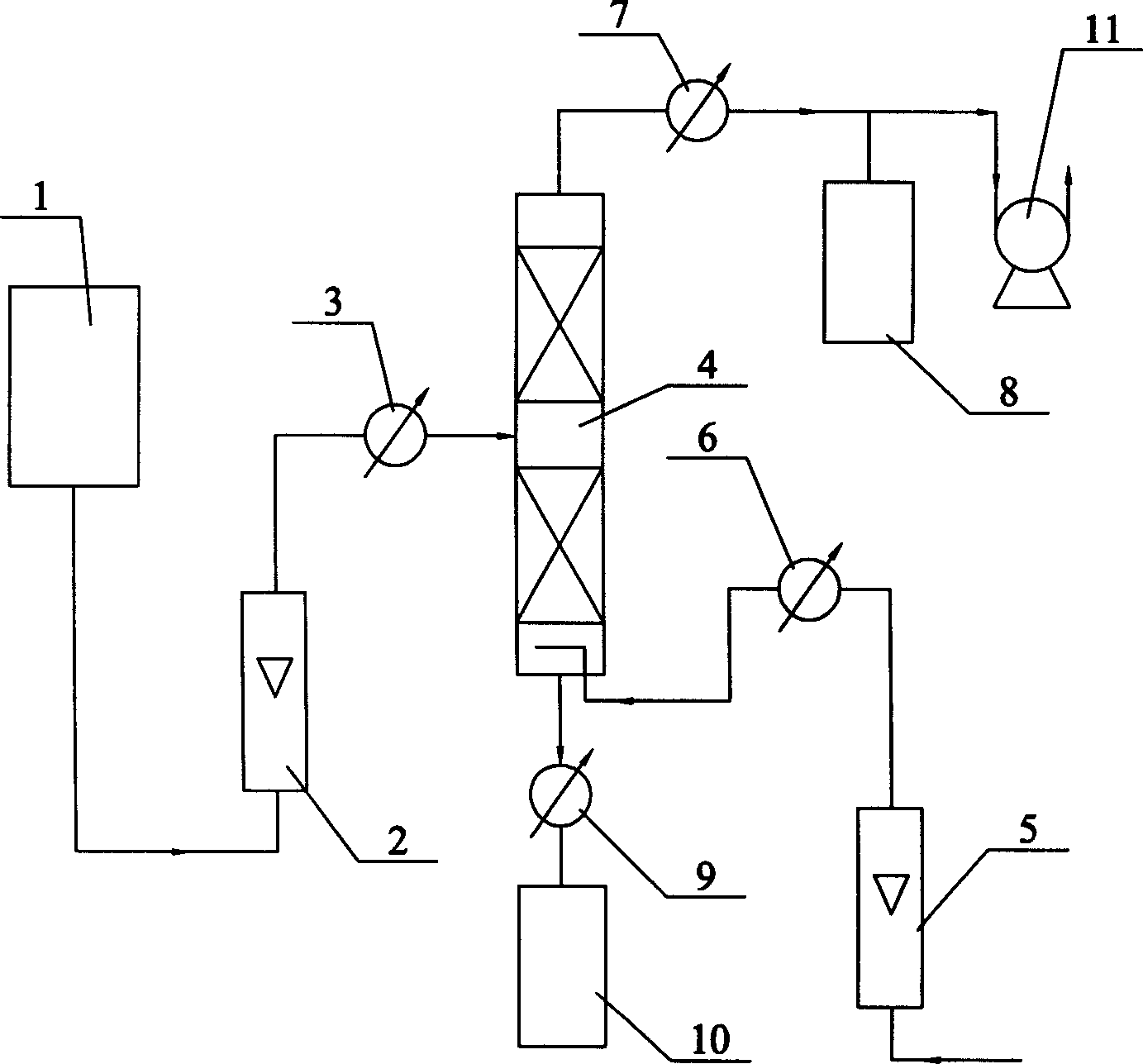
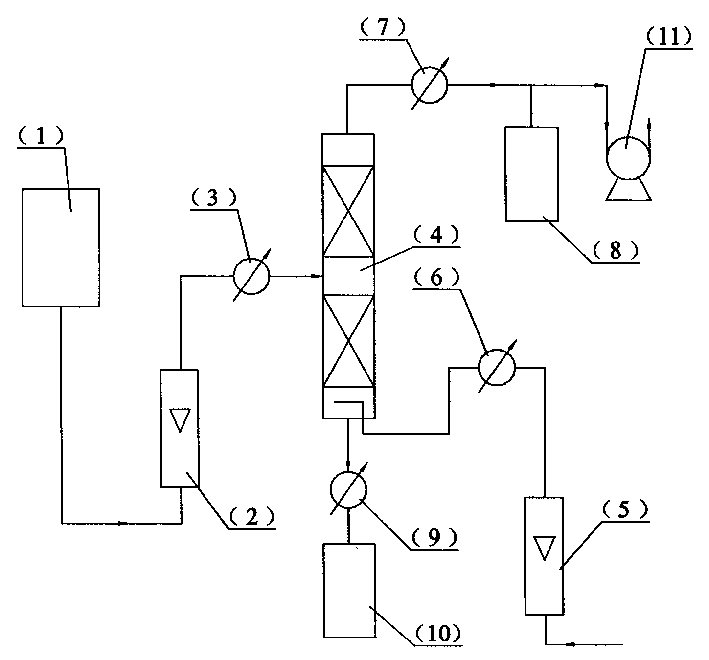
[0005] Specific embodiment one: In this embodiment, the 3-hydroxy propionaldehyde aqueous solution containing 2.9% by weight of acrolein in the acrolein hydration reaction is passed into the raw material tank 1, and the vacuum pump 11 is turned on to make the rectification tower 4 vacuum, and the 3- The aqueous solution of hydroxypropionaldehyde enters the preheater 3 after being measured by the flowmeter 2, and the temperature of the material entering the middle part of the rectification tower 4 after preheating is 55~58°C, so that the superficial liquid velocity of the aqueous solution of 3-hydroxypropionaldehyde is 0.5m / h, the number of trays in the rectifying tower 4 is 17 to 21, the vacuum degree of the rectifying tower 4 is -0.076 to -0.080Mpa, and the air preheated to 55 to 58°C is introduced, the gas-liquid volume ratio The ratio is 10 to 12 times, and the weight content of acrolein in the aqueous solution of 3-hydroxy propionaldehyde after separation is reduced from 2....
specific Embodiment approach 2
[0006] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the 3-hydroxy propionaldehyde aqueous solution containing 3.5% by weight of acrolein in the acrolein hydration reaction is passed into the raw material tank 1, and then enters the middle material of the rectification tower 4 The temperature is 58~62℃, the superficial liquid velocity is 0.6m / h, the number of trays of the rectifying tower 4 is 12~16, and the vacuum degree of the rectifying tower 4 is -0.084~-0.086Mpa. The air heated to 58-62°C has a gas-liquid volume ratio of 14-16 times. After separation, the weight content of acrolein in the 3-hydroxy propionaldehyde aqueous solution is reduced from 3.5% to 0.8%. Its recycling process is the same as the specific embodiment one.
specific Embodiment approach 3
[0007] Specific embodiment 3: This embodiment is different from specific embodiment 1 in that the 3-hydroxypropionaldehyde aqueous solution containing 6.1% by weight of acrolein in the acrolein hydration reaction is passed into the raw material tank 1, and then enters the middle material of the rectification tower 4 The temperature is 62~65℃, the superficial liquid velocity is 0.6m / h, the number of trays of the rectifying tower 4 is 12~16, the vacuum degree is -0.082~-0.084Mpa, and it is preheated to 62~65 In the air at ℃, the gas-liquid volume ratio is 14-16 times. After separation, the weight content of acrolein in the 3-hydroxy propionaldehyde aqueous solution is reduced from 6.1% to 0.8%. Its recycling process is the same as the specific embodiment one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com