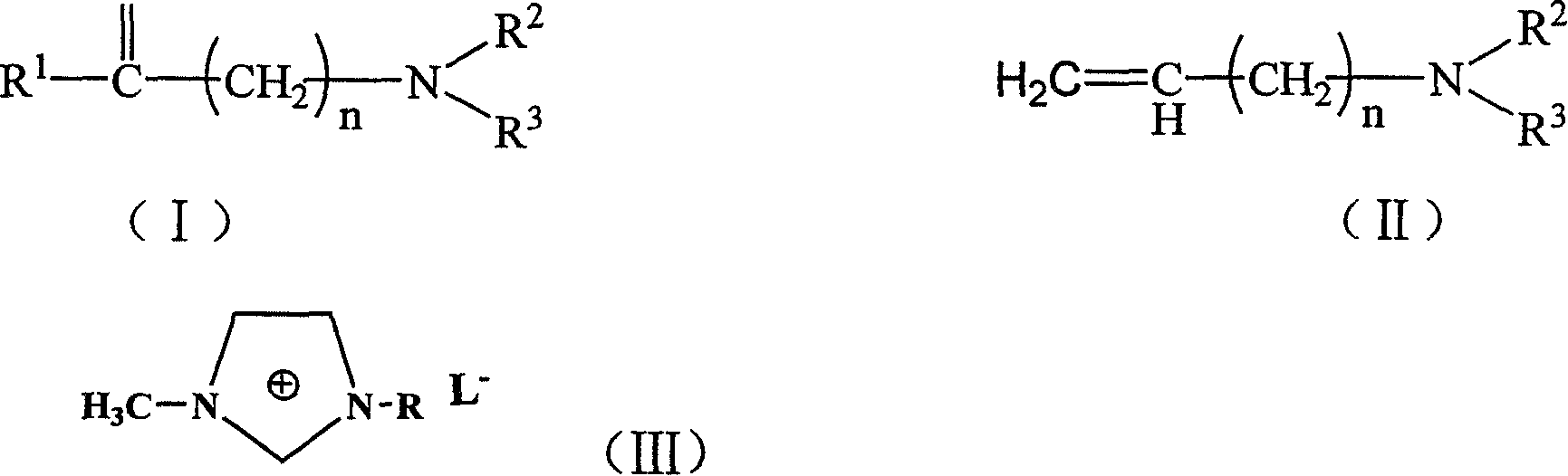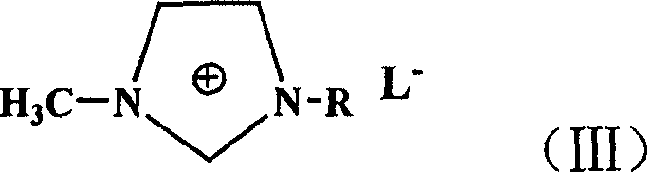Process for preparing 2-substituted enamine or enoyl amine
An enamide and compound technology, which is applied in the field of preparation of arylalkenylamine compounds, can solve the problems of low yield, complex preparation process, environmental pollution and the like, and achieves the effects of high yield, simple preparation process and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of 1-[1-(4-acetylphenyl)vinyl]-2-pyrrolidone
[0029] 199 mg (1 mmol) of 4-bromoacetophenone, 9 mg (0.04 mmol) of palladium acetate, 33 mg (0.08 mmol) of 1,3-bis(diphenylphosphine) propane, N-vinyl- 334 mg (3 mmol) of 2-pyrrolidone, 17 mg (0.16 mmol) of triethylamine, 2 ml of 1-butyl-3-methylimidazolium tetrafluoroborate, placed in a 50 ml three-necked flask, stirred and heated , reacted at 110°C for 15 hours. After the reaction was completed, it was cooled, the reaction solution was separated to remove the ionic liquid, and extracted with dichloromethane to obtain 206 mg of the product, with a yield of 90%.
[0030] 1 H NMR (CDCl 3 )δppm: 7.92~7.94(m, 2H), 7.44~7.41(m, 2H), 5.40(s, 1H), 5.30(s, 1H), 3.60(t, J=7.0Hz, 2H), 2.60(s , 3H), 2.58(t, J=7.5Hz, 2H), 2.16(dd, J=7.0, 7.5Hz, 2H);
[0031] 1 3C NMR (CDCl 3 )δppm: 198.8, 174.8, 143.6, 140.6, 137.3, 128.9, 126.9, 110.4, 49.9, 32.5, 27.0, 19.0;
[0032] MS (m / z): 229 (M + ).
Embodiment 2
[0033] Example 2 Preparation of 10[1-(4-fluorophenyl)vinyl]-2-pyrrolidone
[0034] 175 mg (1 mmol) of 4-fluorobromobenzene, 9 mg (0.04 mmol) of palladium acetate, 33 mg (0.08 mmol) of 1,3-bis(diphenylphosphine)propane, N-vinyl-2 - 334 mg (3 mmol) of pyrrolidone, 17 mg (0.16 mmol) of triethylamine, 2 ml of 1-butyl-3-methylimidazolium tetrafluoroborate, placed in a 50 ml three-necked flask, stirred and heated, React at 125°C for 20 hours. After the reaction was completed, it was cooled, the reaction solution was separated to remove the ionic liquid, and extracted with dichloromethane to obtain 180 mg of the product with a yield of 88%.
[0035] 1 H NMR (CDCl 3 )δppm: 7.92~7.94(m, 2H), 7.06~7.01(m, 2H), 5.30(s, 1H), 5.25(s, 1H), 3.55(t, J=7.0Hz, 2H), 2.56(t , J=8.0Hz, 2H), 2.12(dd, J=7.0, 8.0Hz, 2H);
[0036] 13 C NMR (CDCl 3 )δppm: 174.8, 164.8, 162.6, 143.6, 128.3, 115.9, 109.2, 49.9, 32.3, 19.0;
[0037] MS (m / z): 205 (M + ).
Embodiment 3
[0038] Example 3 Preparation of 1-[1-(4-methoxyphenyl)vinyl]-2-pyrrolidone
[0039]187 mg (1 mmol) of 4-bromoanisole, 9 mg (0.04 mmol) of palladium acetate, 33 mg (0.08 mmol) of 1,3-bis(diphenylphosphine)propane, N-vinyl- 334 mg (3 mmol) of 2-pyrrolidone, 17 mg (0.16 mmol) of triethylamine, 2 ml of 1-butyl-3-methylimidazolium tetrafluoroborate, placed in a 50 ml three-necked flask, stirred and heated , reacted at 115°C for 20 hours. After the reaction was completed, it was cooled, the reaction solution was separated to remove the ionic liquid, and extracted with dichloromethane to obtain 198 mg of the product, with a yield of 91%.
[0040] 1 H NMR (CDCl 3 )δppm: 7.06~6.97(m, 2H), 6.75~6.47(m, 2H), 4.98(s, 1H), 4.87(s, 1H), 3.45(s, 3H), 2.19(dd, J=7.0, 8.0Hz, 2H), 2.10(t, J=8.0Hz, 2H), 1.76(m, 2H);
[0041] 13 C NMR (CDCl 3 )δppm: 172.8, 160.8, 143.6, 127.3, 127.0, 126.8, 114.9, 114.3, 107.2, 55.9, 41.5, 32.8, 19.0;
[0042] MS (m / z): 217 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com