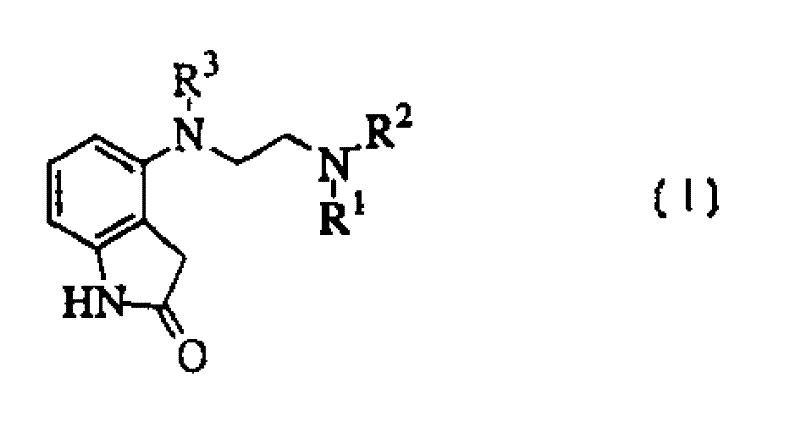
4-amino-(ethylamino)-oxindole dopamine autoreceptor agonists
An alkyl and phenyl technology, applied in the field of 4-amino-oxindole, can solve the problems such as no disclosure or suggestion of receptor agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] 4-(2-Benzylamino-ethylamino)-1,3-dihydro-indol-2-one Dihydrochloric acid
[0090] Salt 0.1 water
[0091] N-[2-(Benzyl-trifluoroacetylamino)ethyl]-2,2,2-trifluoro-N-(2-oxo-2,3-dihydro-1H-indole-4- Base)-acetamide (1.0 g, 2.1 mmol) was dissolved in 40 ml of tetrahydrofuran, and at room temperature, a solution formed by dissolving 50% sodium hydroxide (1.0 ml) in 10 ml of methanol was added. After 15 minutes, the mixture was concentrated and the residue was dissolved in 50 mL of ethyl acetate. The organic layer was washed with 2 x 50ml of water, 50ml of brine, dried over anhydrous magnesium sulfate, and filtered. Purification by silica gel chromatography (7% 2N ammonia methanol-ethyl acetate), crystallization from ethyl acetate gave the free base as a light green solid (0.7 g, 87.5%), mp 120-124°C; MS EI m / e 281 ( m + ).
[0092] C 17 h 19 N 3 O 0.25H 2 Elemental analysis of O
[0093] Calculated: C, 71.43; H, 6.88; N, 14.70
[0094] Found va...
Embodiment 2
[0100] 4-[2-(Benzyl-methyl-amino)-ethylamino]-1,3-dihydro-indol-2-one·di
[0101] Hydrochloride·0.8 water
[0102] N-[2-(Benzyl-methyl-amino)ethyl]-N-(3-chloro-1H-indol-4-yl)-2,2,2-trifluoroacetamide (5b, 1.75 g , 4.27mmol) was dissolved in 27ml of acetic acid to form a solution, and 70% phosphoric acid (20ml) was added. The reaction was heated at 75-80°C for 16 hours. The mixture was poured into 100ml of water and the crude product crystallized out as a solid. After filtration, the solid was dissolved in 100 ml of ethyl acetate*, washed with 50 ml of water, 50 ml of saturated sodium bicarbonate solution and 20 ml of brine, dried over anhydrous magnesium sulfate, and filtered. Concentration with ethyl acetate gave crude intermediate 6b as a dark green residue (1.5 g). Without further purification, this material was dissolved in 40 ml of tetrahydrofuran, and a mixture of 1.0 ml of 50% sodium hydroxide dissolved in 10 ml of methanol was added at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com