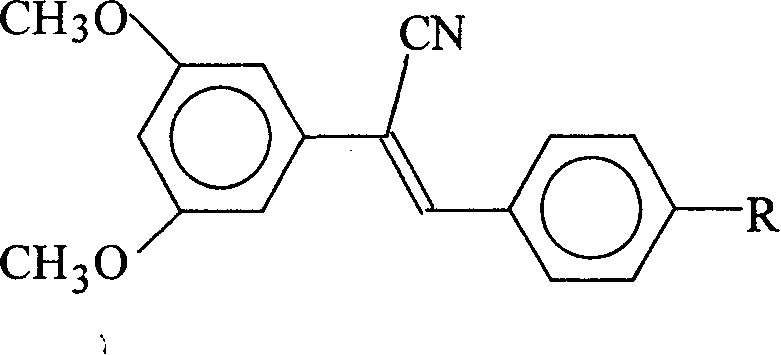
1-cyan-1-(3,5-dimethoxy phenyl)-2-(4-R group phenyl) ethene and preparation method
A technology of dimethoxyphenyl and phenyl phenyl, applied in the fields of organic chemistry and medicinal chemistry, can solve the problems of inability to complete, the effect of resveratrol is not strong enough, and cell apoptosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of 1-cyano-1-(3,5-dimethoxyphenyl)-2-(4-methoxyphenyl)ethene
[0033] 3, the preparation of 5-dimethoxyphenylacetonitrile (1):
[0034] Add 9.8 grams of sodium cyanide and 50 milliliters of water into a 250 milliliter three-necked flask, stir to dissolve the solid, add 50 milliliters of absolute ethanol, raise the temperature to 65°C, add 40 grams of 3,5-dimethoxybenzyl bromide, and The reaction was incubated for 1.5 hours. Pour out the reaction solution, cool to room temperature and precipitate a solid, filter to obtain a crude product, recrystallize from methanol / water to obtain 23 g of white needle-like crystals, melting point 51-53°C.
[0035] Preparation of 1-cyano-1-(3,5-dimethoxyphenyl)-2-(4-methoxyphenyl)ethene (2a):
[0036] In a 150 ml three-necked flask, 3.54 g of 3,5-dimethoxyphenylacetonitrile (1), 2.72 g of p-methoxybenzaldehyde and 20 ml of methanol were added. Stir and heat up to 60°C, and add 1 g of sodium methoxide. The react...
Embodiment 2
[0037] Example 2: Preparation of 1-cyano-1-(3,5-dimethoxyphenyl)-2-phenylethylene (2b)
[0038] Using a method similar to Example 1, by 3.54 grams of 3,5-dimethoxyphenylacetonitrile (1) and 2.12 grams of benzaldehyde and 20 milliliters of methanol, 1 gram of sodium methylate, react to obtain 1-cyano-1- (3,5-dimethoxyphenyl)-2-phenylethene (2b) 2.4 g, melting point 68-70°C.
Embodiment 3
[0039] Example 3: Preparation of 1-cyano-1-(3,5-dimethoxyphenyl)-2-(4-nitrophenyl)ethene (2c)
[0040] Adopt the similar method of embodiment 1, by 3.54 grams of 3,5-dimethoxyphenylacetonitrile (1) and 3 grams of 4-nitrobenzaldehyde and 20 milliliters of methanol, 1 gram of sodium methylate, react to obtain golden yellow crystals 5.5 g of 1-cyano-1-(3,5-dimethoxyphenyl)-2-(4-nitrophenyl)ethene (2c), melting point 214-216°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com