Cyclodextrin grafted biocompatible amphiphilic polymer and methods of preparation and use thereof
A technology of biocompatible and hydrophilic polymers, applied in the direction of non-active ingredients of polymer compounds, medical preparations of non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] Materials and methods : PEG with propionic acid pendant groups (PEG-10PA and PEG-8PA Mw=~20KD, SunBio Company, Anyang City, South Korea) was dried overnight at room temperature under vacuum. β-Cyclodextrin (TCI (USA), Portland, OR) was dried overnight at 130° C. under vacuum before use. Other chemicals were from Aldrich Chemical Company (Milwaukee, WI) and used as supplied without further purification. HPLC (High Pressure Liquid Chromatography) analysis was performed on a Waters system equipped with RI detector and Ultrahydrogel 120 and Ultrahygel 500SEC columns. 1 H-NMR was recorded on a Varian 400 MHz machine.
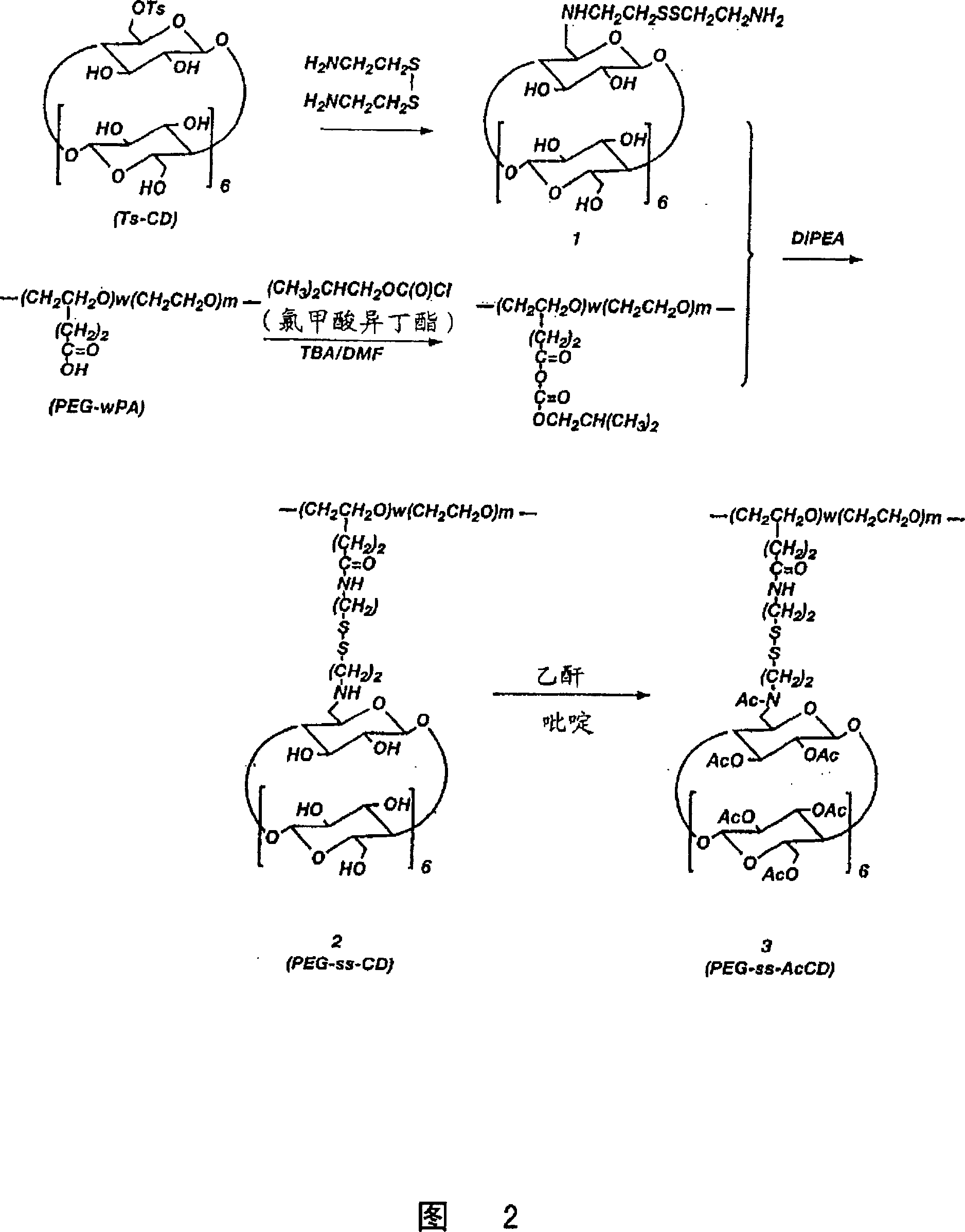
[0070] PEG-SS-CD Synthesis of (Compound 2)
[0071] Mono-6-(6-amino-3,4-dithio-hexylamino)-6-deoxy-β-cyclodextrin (Compound 1):
[0072] 2,2'-Dithiodiethylamine dihydrochloride (10 g, 4.44 mol, Fw=225.2) was dissolved in 30 mL of distilled water, followed by the addition of 1.0 M KOH (8.88 mol) and mono-6-toluenesulfonyl-β- Cyclodextrin (0.5 g, Fw=128...
example 2
[0077] PEG-SS-AcCD Synthesis of (Compound 3)
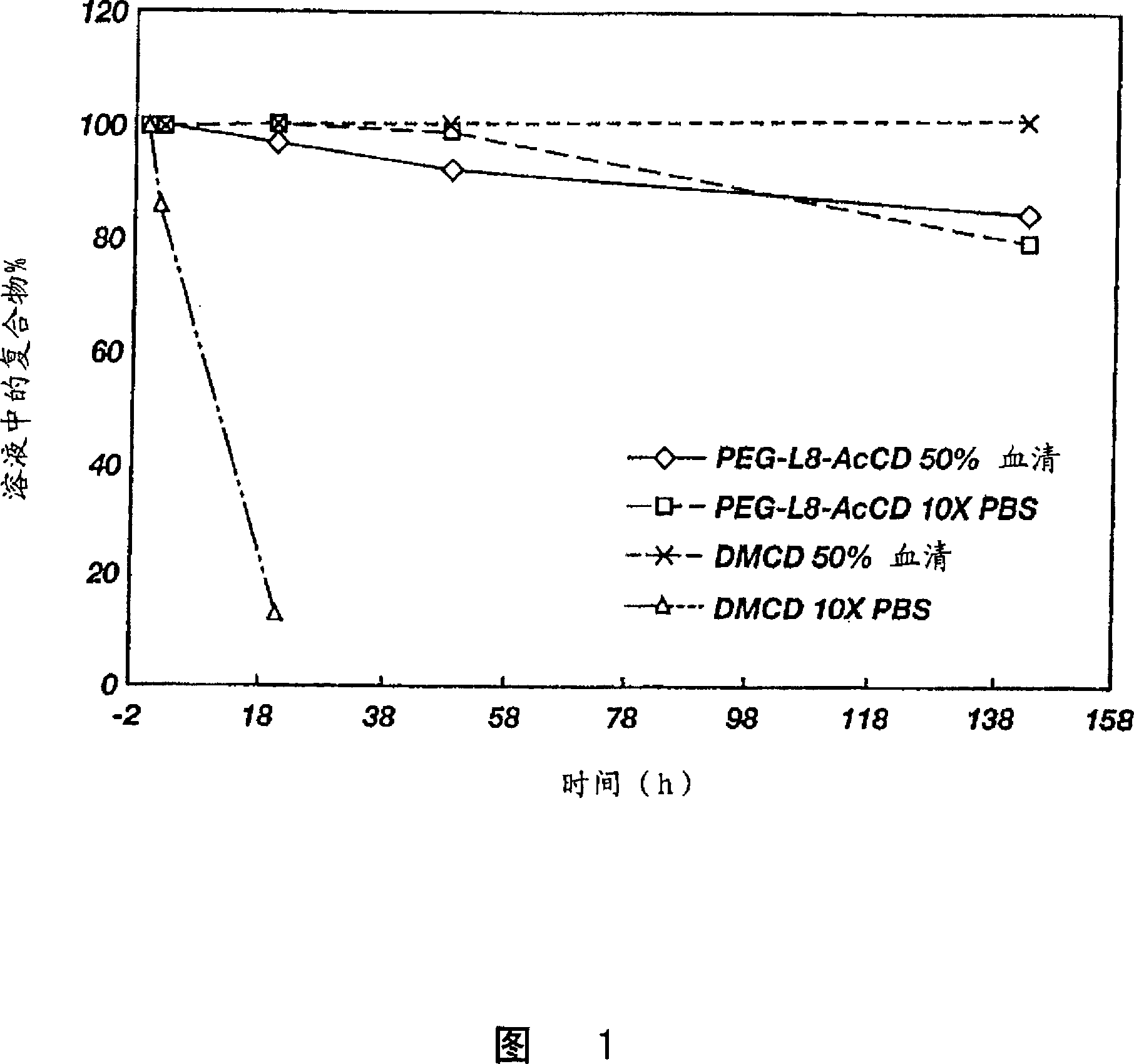
[0078] PEG-SS-CD (compound 2, 1.0g, about 5CD / 20 KD-PEG) in P 2 o 5 Dry in a desiccator, then co-evaporate with 50 mL of anhydrous pyridine. The residue was dissolved in 30 mL of pyridine under the protection of argon, and then added to 2.0 mL of acetic anhydride (Fw=102.1, d=1.08). The mixture was stirred at room temperature for 2 days and dried on a rotary evaporator. The crude product was purified by repeated ether precipitation from methanol. HPLC (GPC) analysis showed that the product was longer than the base polymer by 0.46 min compared to the base polymer (Rt = 19.70 min for the product vs. Rt = 19.24 min for the reactant polymer). 1 H-NMR analysis indicated about 5 CD moieties per 20KD PEG and all hydroxyl groups were acetylated.
[0079] 1 H-NMR (400MHz, D 2 O): δ, 4.7-5.5(s, 14H, H1', H3'), 3.4-5.5(m, 382H, 35H-CD, 347H-PEG), 2.05(m, 20H, H-Ac).
example 3
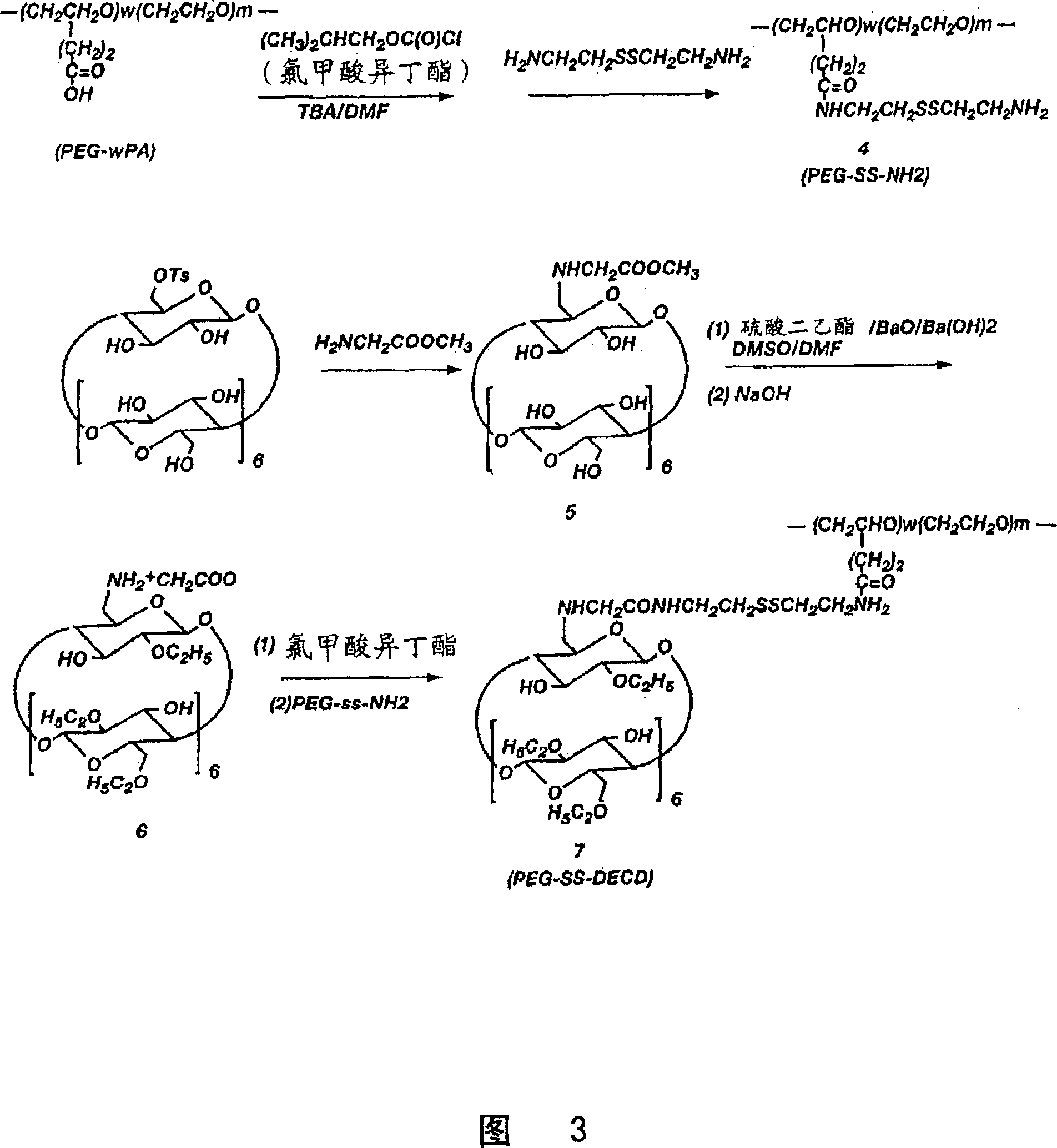
[0081] PEG-SS-DECD Synthesis of (compound 7)
[0082] PEG-SS-NH2 (Compound 4):
[0083] PEG grafted with carboxyl groups (PEG-8PA, 2.6 g, about 2.0 mmol COOH groups) was dissolved in 30 mL of anhydrous DMF and cooled on ice to 0 °C. Tributylamine (0.35 mL, 1.5 mmol, Fw=185.36, d=0.778) was added thereto, followed by isobutyl chloroformate (0.20 mL, 1.5 mmol, Fw=136.6, d=1.053). The mixture was stirred at 0°C for 80 min, then carefully added to a solution of 2,2'-dithiodiethylamine (3.5 g, Fw = 152.2, 23 mmol) in 50 mL of anhydrous DMF. The mixture was stirred at room temperature for 20 h, concentrated to about 20 mL at 40 °C on a rotary evaporator, then dialyzed against distilled water after dilution with 50 mL of water (4 x 5 L, 26 h, Sigma D-0655, MWCO=12,000). The dialysis solution was concentrated by rotary evaporation at 40°C, resulting in 4.1 g of slurry. The slurry was dissolved in 10 mL of methanol and subsequently precipitated by adding 80 mL of ethyl ether. T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com