Preparing method for cytarabine
A technology of cytarabine and arabinoside, which is applied in the field of preparation of nucleotide drug cytarabine, can solve the problems of high price, increased production cost, and high price of pyridine, and achieve low price, low quantity, and control The effect of high production cost and production yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
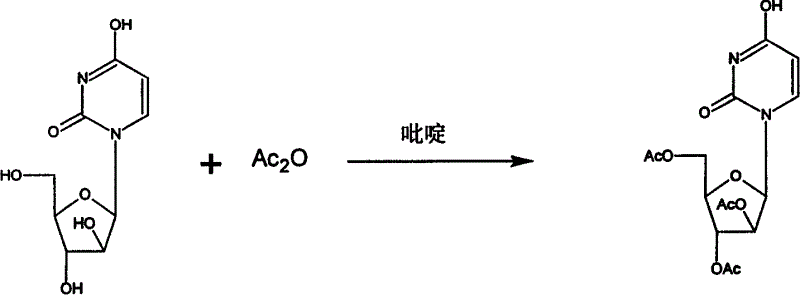
[0037] Example 1: Preparation of ethyl arabinouridine (compound VII):
[0038] Add 26.2 kg of pyridine to the reaction tank, stir, add 11.5 kg of arabinouridine and 18.6 kg of acetic anhydride in sequence, and stir for 3 hours at room temperature. After the reaction was completed, 11.5 liters of methanol was added, stirred for 0.5 hours, and concentrated under reduced pressure until the pyridine could not be evaporated. Add ethanol, stir and heat to dissolve. Cool to below 0°C overnight. After discharging, filtering with suction, and drying, 15.8 kg of compound VII was obtained with a yield of 91%.
Example Embodiment
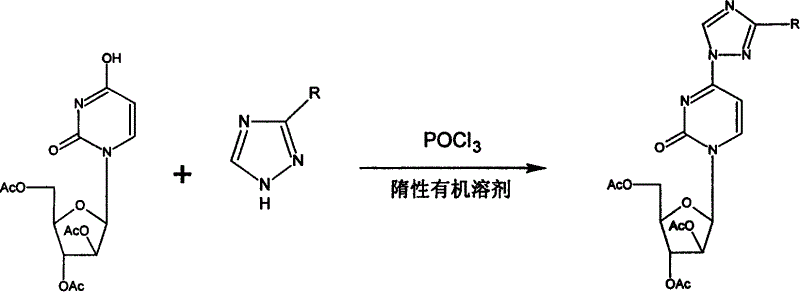
[0039] Example 2: Preparation of Uridine Triazole (Compound IX):
[0040] Add 150L of chloroform into the reaction tank, stir, and add 15kg of triazole. Cool to below 5°C and add 9.225kg of phosphorus oxychloride. After adding phosphorus oxychloride, continue stirring to below 5°C, and add 33.75L of triethylamine dropwise. Control the dripping acceleration so that the maximum temperature rise does not exceed 8°C. Add 15 kg of ethyl arabinouridine (compound VII) into the above reaction tank. Stir at room temperature overnight. When the reaction is complete, add 150L of water and stir to release the chloroform layer. The aqueous phase was extracted twice with chloroform 60L×2, the organic phase was combined with the former chloroform layer, and dried with anhydrous magnesium sulfate. Suction and filter to remove magnesium sulfate, the filtrate is concentrated to dry chloroform and then added with absolute ethanol, stirred and refluxed for dissolution. Cool to below 0°C overnight. Af...
Example Embodiment
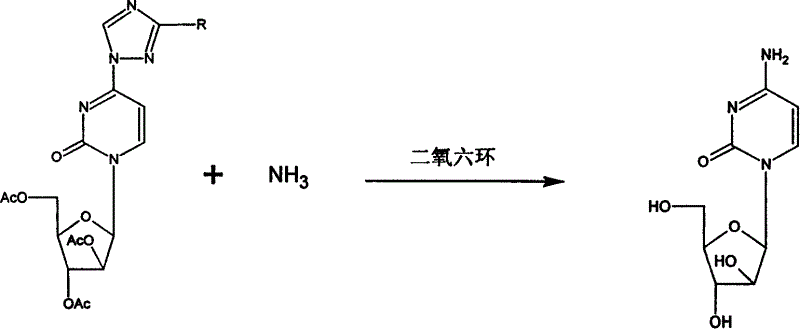
[0041] Example 3: Preparation of Cytarabine:
[0042] Add 68.5 kg of dioxane into the reaction tank, stir and cool to 15°C, and vent ammonia for 5-6 hours. Add 13.7 kg of uridine triazole (compound X) and 9.6 kg of ammonia, and stir overnight at room temperature. The dioxane was concentrated under reduced pressure until no more liquid distilled out. 54.8kg methanol ammonia was added and reacted overnight. After the reaction, it was concentrated to dryness under reduced pressure. Dissolve the residue in 60L of non-ionized water and load 001X4 ion exchange resin. First rinse with deionized water, then eluted with 5% diluted ammonia water, collect the product-containing eluate, and concentrate it under reduced pressure to dryness as a crude product. The crude product was dissolved in water and ethanol under reflux. Filter while it is hot, and the filtrate enters the fine drying package crystallization tank. Crystallize overnight. After suction filtration, the crystals are filtered ou...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap