Puerarin prepn for eye and its prepn process
An ophthalmic preparation, puerarin technology, applied in the field of puerarin ophthalmic preparations, can solve the problems of high viscosity of hydrophilic gel, inaccurate dosage, long residence time, etc., to improve bioavailability, accurate dosage, The effect of prolonging the residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0022] Example 1 The best choice of the hydroxypropyl methylcellulose model of the pharmaceutical excipient of the present invention
[0023] Hydroxypropyl methyl cellulose is an auxiliary thickening agent. The following is the thickening effect of hydroxypropyl methyl cellulose of different specifications on 1 part of puerarin. The results are shown in Table 1.
[0024] Table 1 Investigation of the thickening effect of different dosages and different types of hydroxypropyl methylcellulose on 1 part of puerarin (mpa.s)
[0025] Number of copies / model E3 E15 E50 E100 E4M E10000
[0026] 0.1 part / / / 5 25 80
[0027] 0.5 copies / / 20 10 75 200
[0028] 1.5 copies / / 50 40 150 /
[0029] 2.0 copies 5 20 80 120 / /
[0030] 2.5 servings 10 30 150 / / /
[0031] 10 servings 40 100 / / / /
[0032] 100 servings 200 / / / / /
[0033] From the above table using different types of hydroxypropyl methyl cellulose to investigate the results of different concentrations, it can be seen tha...
Example Embodiment
[0034] Example 2 The best choice of carbomer model
[0035] Prescription
[0036] Table 2 Sample A (Carbomer 940 as the matrix) Viscosity change measurement results when pH changes
[0037] pH 4.02 4.53 5.06 5.48 6.00 6.44 6.95 7.41
[0038] Viscosity (cp) 75 150 275 975 1900 2275 2350 2400
[0039] It can be seen that the sample prepared with Carbomer 940 as the gel matrix has little change in viscosity when the viscosity is 4.56.5, the viscosity changes little.
[0040] Table 3 Sample B (Carbomer 974 as substrate) Viscosity change measurement results when pH changes
[0041] pH 4.09 4.59 5.02 5.51 5.96 6.47 6.98 7.49
[0042] Viscosity (cp) 150 375 650 1100 1450 1750 1825 1900
[0043] It can be seen that the viscosity of the sample prepared with Carbomer 974 as the gel matrix increases continuously when the viscosity is 4.5
[0044] Due to the characteristics of the ...
Example Embodiment
[0045] Example 3 Selection of the best ratio of carbomer and hydroxypropyl methylcellulose
[0046] The viscosity of the samples prepared with Carbomer 940 before and after the phase change using the above test is acceptable. In order to obtain the best prescription, we will examine the dosage of carbomer and the dosage and type of hydroxypropyl methylcellulose. We design as follows The prescriptions are examined, see Table 4.
[0047] Prescription 1
[0048] A series of samples were prepared according to the above process, and the changes in viscosity of each cube were investigated when the pH was 4.5, 5.0 and 7.4. The results are shown in Table 5.
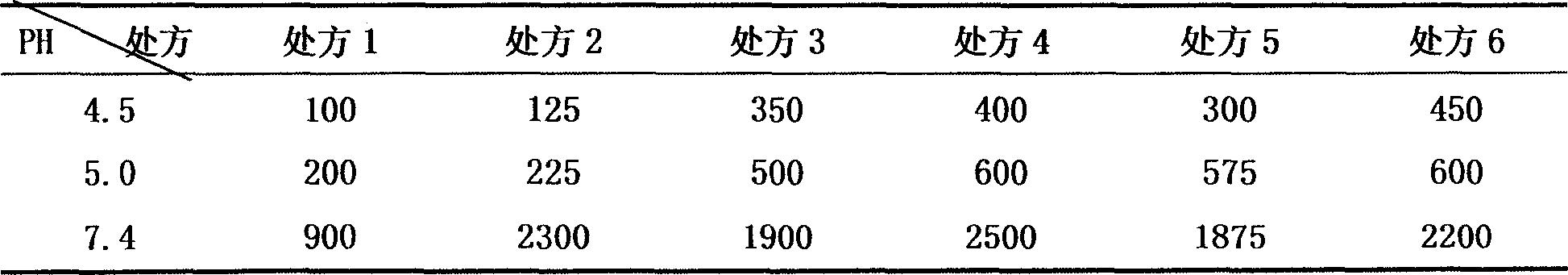
[0049] Table 5 Viscosity change measurement results (mpa.s)
[0050]
[0051] From the above investigation results, we can see that when we change the model and dosage of carbomer 940 and hydroxypropyl methylcellulose, the viscosity of the sample before and after gelation has a big difference. Pre-gelat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap