Method for supplying reaction gases in catalytic vapor phase oxidation process
一种反应气体、氧化反应的技术,应用在有机化学方法、化学仪器和方法、有机氧化等方向,能够解决没有进行任何明确说明等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
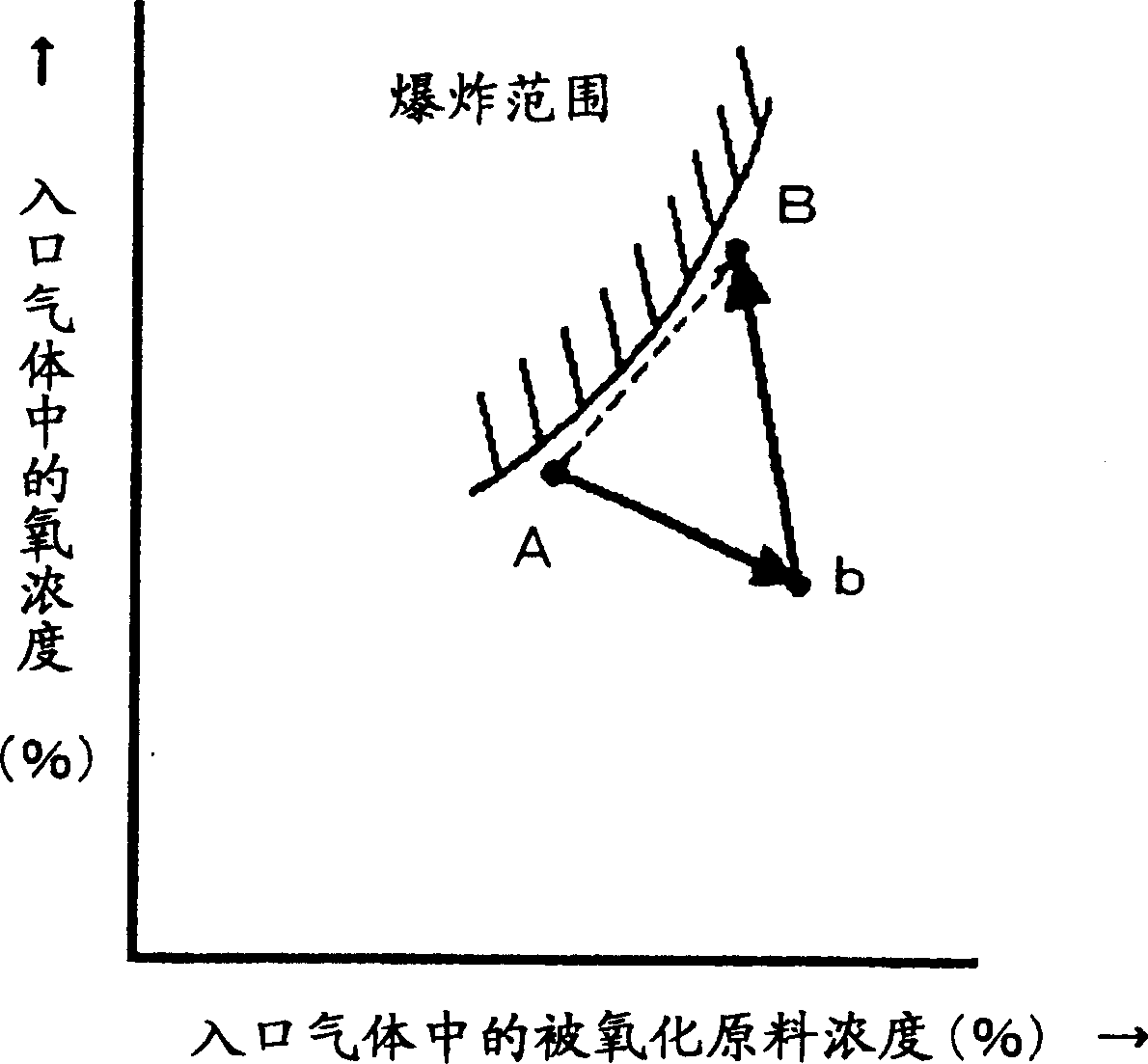
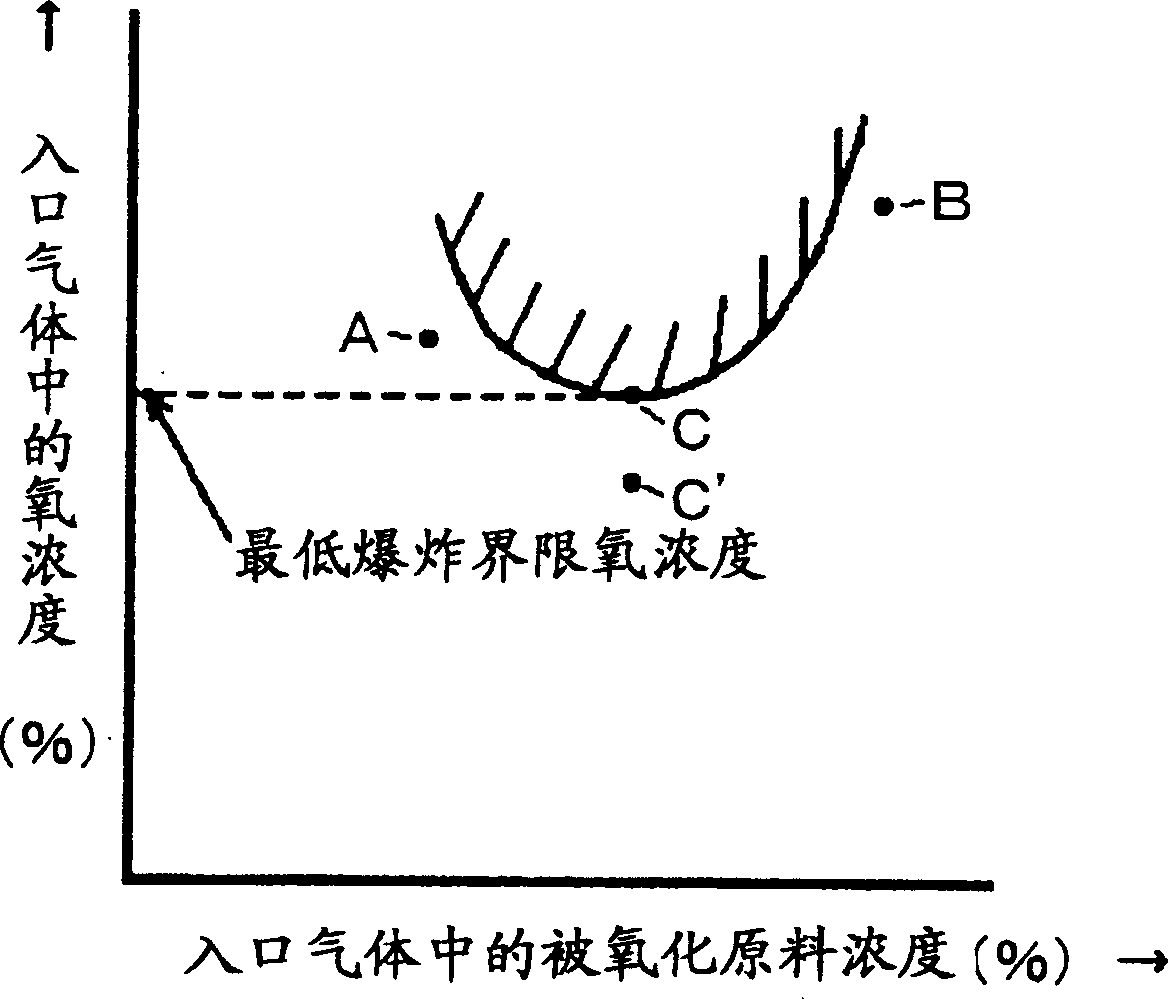
[0040] First, use experimental equipment to mix isobutylene, oxygen, and nitrogen at various composition ratios, and collect experimental data for composition points where explosion occurs and composition points where explosion does not occur. This experimental data was input into the computer equipped with the above-mentioned program, and it was set so that the explosion range of isobutylene and oxygen could be displayed on the monitor.
[0041] Next, in the production process of producing methacrolein by reacting isobutene with oxygen, isobutene, air, and exhaust gas from the capture process are mixed, and the Figure 7 The mixed gas (point A) adjusted so that the concentration of isobutene is 3.5% by volume and the concentration of oxygen is 11% by volume is supplied to the inlet of the oxidation reactor. The exhaust gas from the trapping process is used as a dilution gas for adjusting the composition. From this state, the operating load was changed to an isobutylene conce...
Embodiment 2
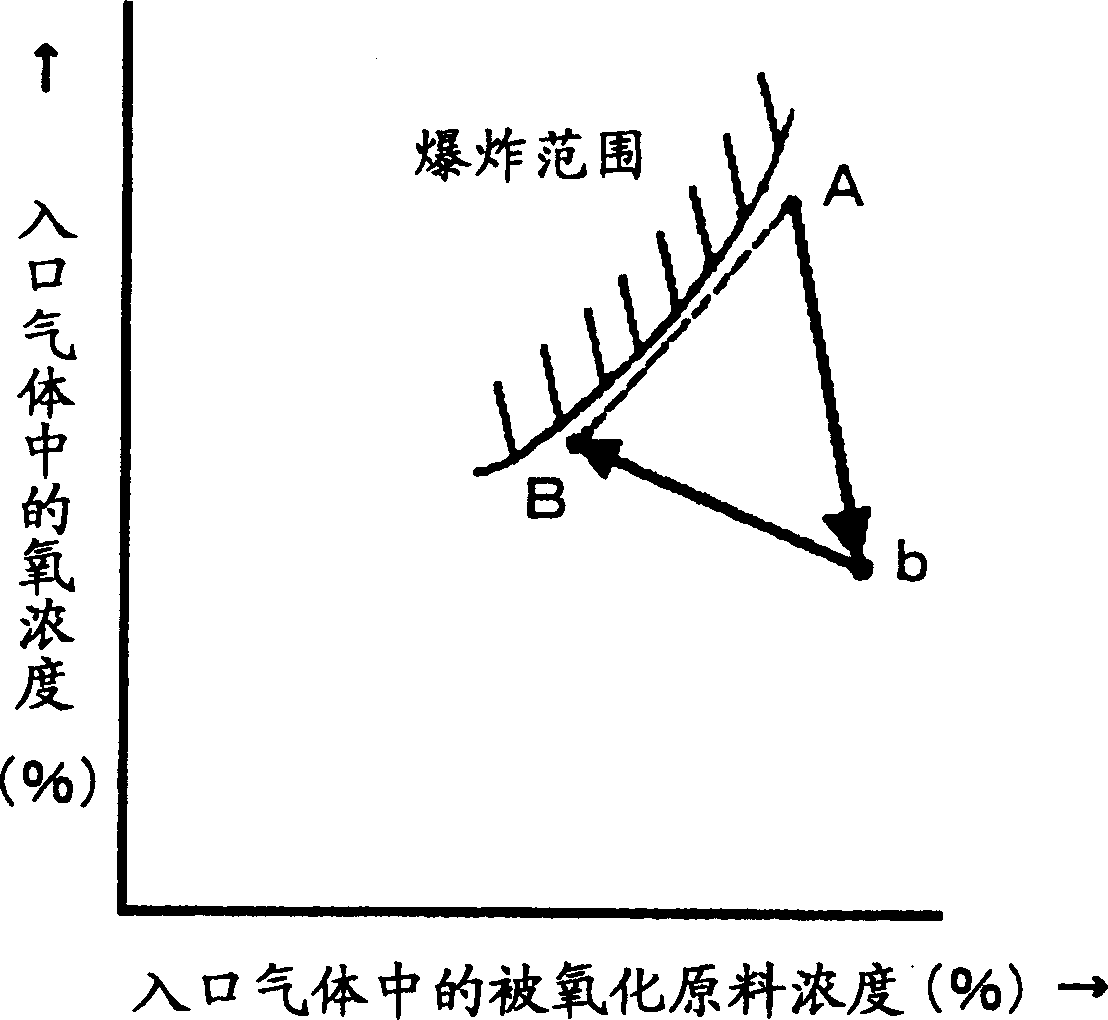
[0043] First, use experimental equipment to mix methacrolein, oxygen, and nitrogen at various composition ratios, and collect experimental data on composition points where explosion occurs and composition points that do not explode. Input the experimental data into the computer equipped with the above program, and set it so that the outbreak range of methacrolein and oxygen can be displayed on the monitor.
[0044] Next, in the production process of producing methacrylic acid by reacting methacrolein with oxygen, methacrolein, air, and exhaust gas from the capture process are mixed, and the Figure 8 A mixed gas (point A) having an isobutene concentration adjusted to 3.5% by volume and an oxygen concentration adjusted to 9.65% by volume as shown was supplied to the inlet of the oxidation reactor. The exhaust gas from the trapping process is used as a dilution gas for adjusting the composition. From this state, the operating load was changed to a methacrolein concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com