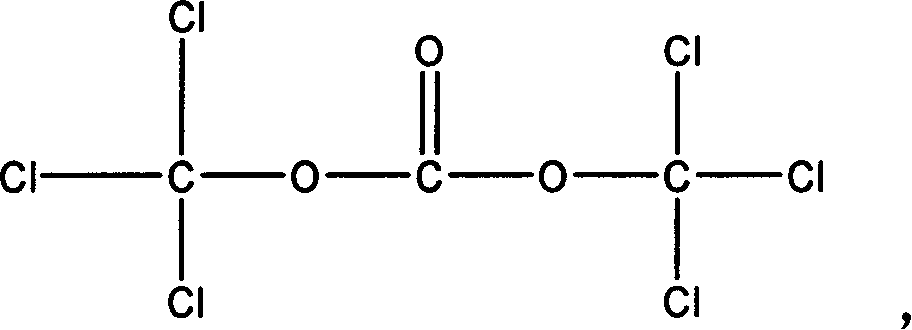
Prepn of isocyanate-containing alkyl silane or alkyl siloxane
A technology of cyanate alkoxysilane and isocyanate alkylsilane, which is applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve complex reaction process operations, pollute the environment, Low safety and other issues, to achieve the effect of simple process route, cheap and easy-to-obtain raw materials, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0049] Example one, the synthesis of isocyanate propyltriethoxysilane:
[0050] 1. Dissolve 20 grams of bis(trichloromethyl)carbonate into 150ML of tetrahydrofuran solution and add it to the constant pressure dropping funnel A;
[0051] 2 Add 44 grams of aminopropyltriethoxysilane and 70 grams of pyridine to 60 mL of tetrahydrofuran into the constant pressure dropping funnel B;
[0052] 3. At 0°C ~ 5°C, slowly drop the mixture of A and B into a 500ml four-neck flask respectively, after 2 hours, continue to react at 0°C for 2 hours;
[0053] 4. Raise the temperature to 65°C for 1 hour;
[0054] 5. Reduce to room temperature, filter and remove tetrahydrofuran;
[0055] 6. Finally, bp 130°C / 20mmHg, distilled under reduced pressure to obtain isocyanate propyltriethoxysilane product, 36.6g, with a yield of 70% and a product content of 98.15%.
[0056] Product is analyzed by gas chromatography: product purity 98.15%; FT-IR infrared spectrometer and 1 H-NMR mass spectrometry anal...
Embodiment 2
[0057] Embodiment two, the synthesis of isocyanate propylmethyldiethoxysilane:
[0058] 1. Dissolve 20 grams of bis(trichloromethyl)carbonate into 150ML of tetrahydrofuran solution and add it to the constant pressure dropping funnel A;
[0059] 2. Add 38.2 g of aminopropylmethyldiethoxysilane, 70 g of pyridine and 80 mL of tetrahydrofuran into the constant pressure dropping funnel B;
[0060] 3. At -5°C to 0°C, slowly drop the mixtures of A and B into a 500ml four-neck flask respectively, and the dropwise addition is completed in 2 hours, then continue to react at -5°C to 0°C for 2 hours;
[0061] 4 Heat up to 60°C for 1 hour
[0062] 5. Reduce to room temperature, filter and remove tetrahydrofuran;
[0063] 6. Finally, 29.50 g of isocyanate propylmethyldiethoxysilane was obtained by vacuum distillation at bp91-93°C / 9mmHg, with a yield of 68% and a content of 97.60%.
[0064] The product is analyzed by gas chromatography: product purity 97.60%; FT-IR infrared spectrometer a...
Embodiment 3
[0065] Embodiment three, the synthesis of isocyanate propyltriethoxysilane:
[0066] 1. Dissolve 20 grams of bis(trichloromethyl)carbonate and 150ML of toluene solution into the constant pressure dropping funnel A;
[0067] 2. Add 44 grams of aminopropyltriethoxysilane and 50 grams of dimethylaniline to 60ML of toluene into the constant pressure dropping funnel B;
[0068] 3. At 0°C ~ 5°C, slowly drop the mixture of A and B into a 500ml four-neck flask respectively, after 2 hours, continue to react at 0°C for 2 hours;
[0069] 4. Raise the temperature to 65°C for 1 hour;
[0070] 5. Cool down to room temperature, filter and remove toluene;
[0071] 6. Finally, bp 130°C / 20mmHg vacuum distillation to obtain isocyanate propyltriethoxysilane product, 31.47g, yield 64%, product content 97.92%.
[0072] Product is analyzed by gas chromatography: product purity 97.92%; FT-IR infrared spectrometer and 1 H-NMR mass spectrometry analysis and characterization: at 2272cm-1 is the char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com