Serum-free VERO cell banking process
一种无血清、无血清培养基的技术,应用在动物细胞、细胞培养基、生物化学设备和方法等方向,能够解决组成改变、妨碍产物纯化、朊病毒污染等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
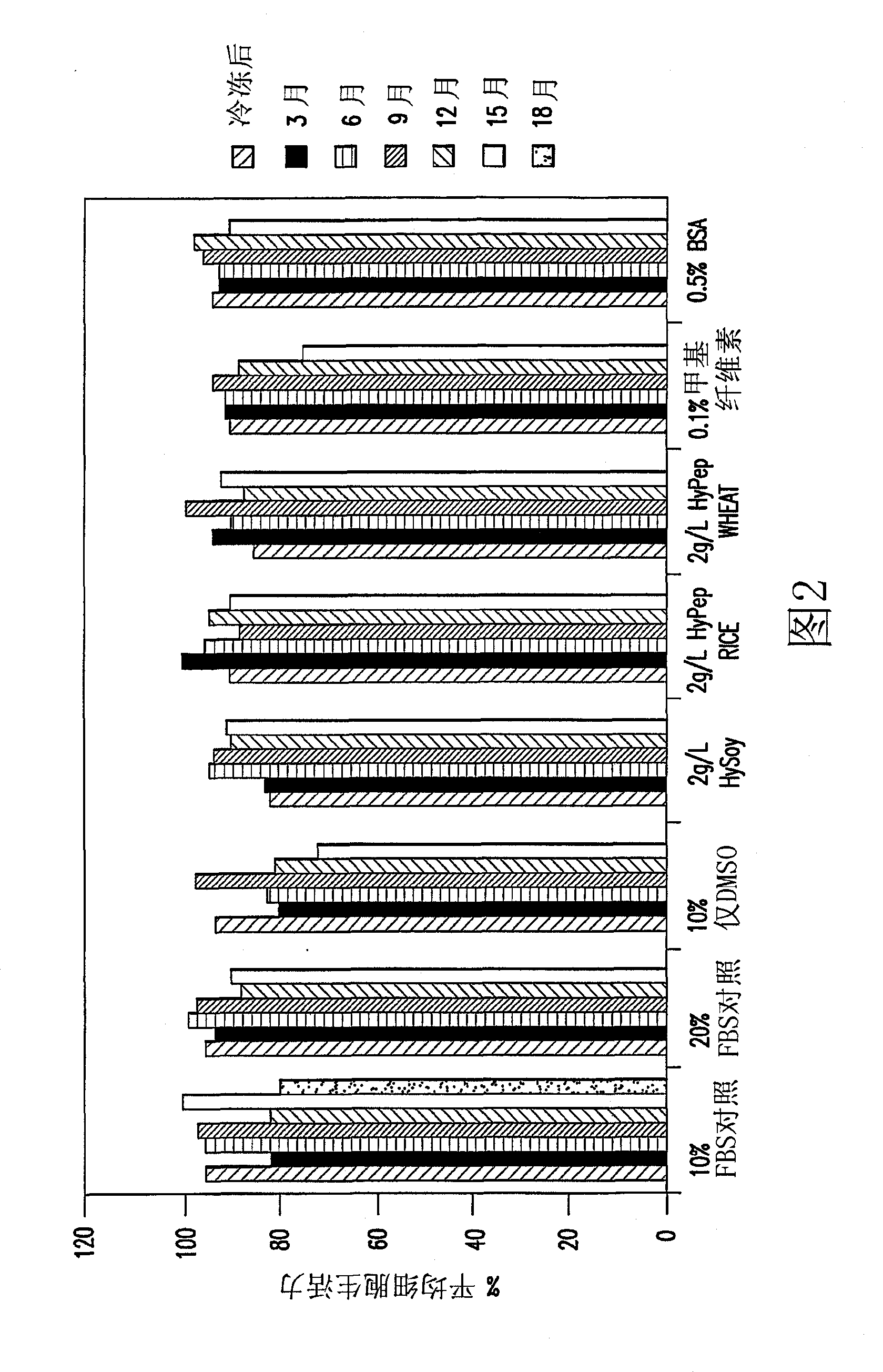
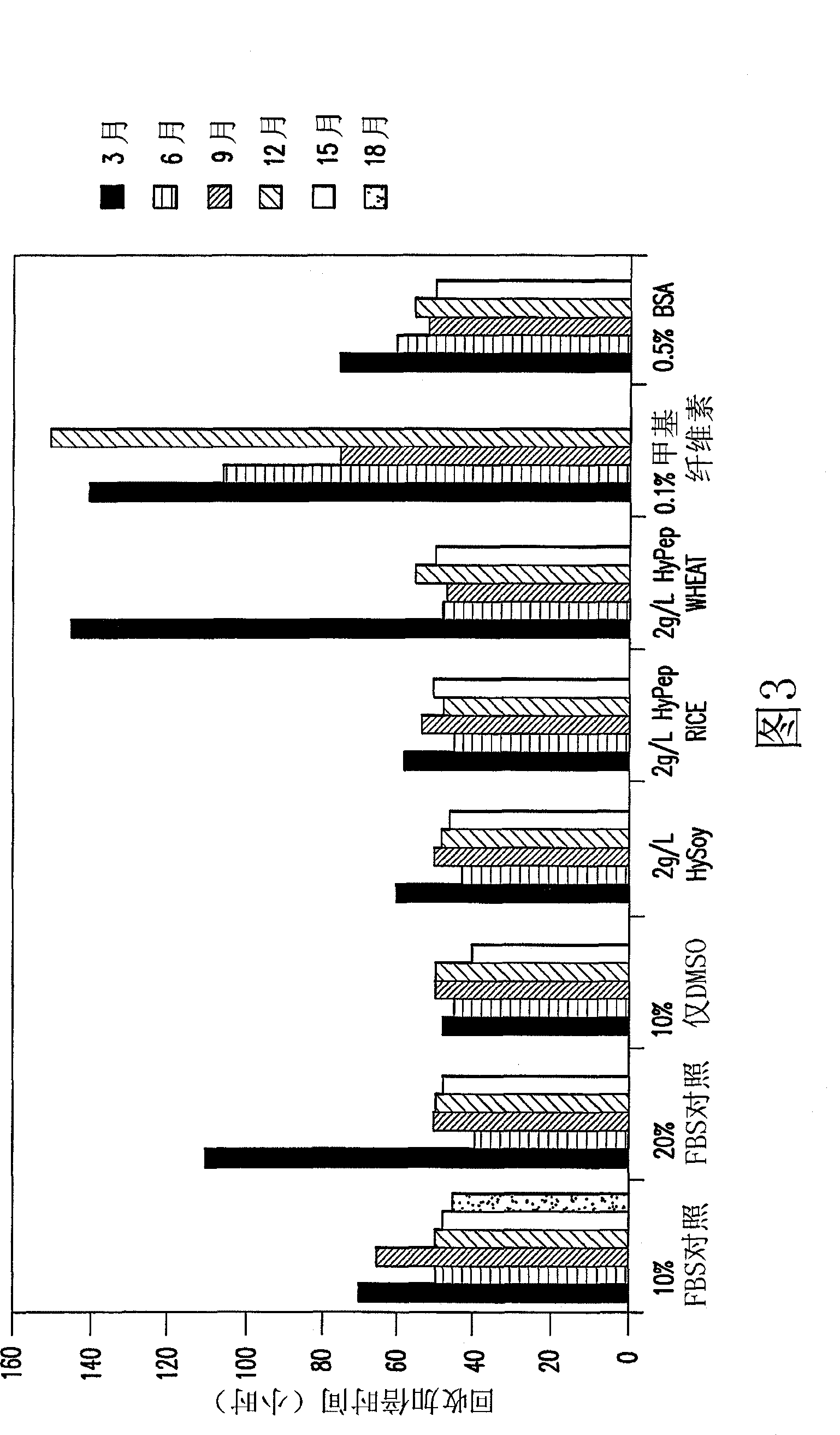
[0035] Several agents were tested as low temperature stabilizers in this study. Cell banks containing Hy-Soy, HyPep Rice, HyPep Wheat, and methylcellulose were compared to cell banks containing FBS and tested for cell bank stability. Hy-Soy and HyPep Rice proved to be as effective as FBS as low temperature stabilizers for the first year of the study. Thus, Hy-Soy can be used in serum-free cell banks and HyPep Rice can be used as a substitute.
[0036] Materials and methods
[0037] Personal protective equipment: sterile gloves, sterile cuffs, sterile coveralls, clean room hoods, clean room beards, and clean room foot covers.
[0038] Disposable Sets: Disposable Serological Pipettes; 1.8ml Sarstedt Cryotubes; Corning Coastar 75cm 2 and 150cm 2 Tissue Culture Flask; Ten-Disc Nunc Cell Factory [www.nuncbrand.com].
[0039] Glassware and Accessories: Glass cover with C-bend for aliquoting media from 10L and 20L total media; Gelman filter with C-bend; Cell Factory adapter; wit...
example 1
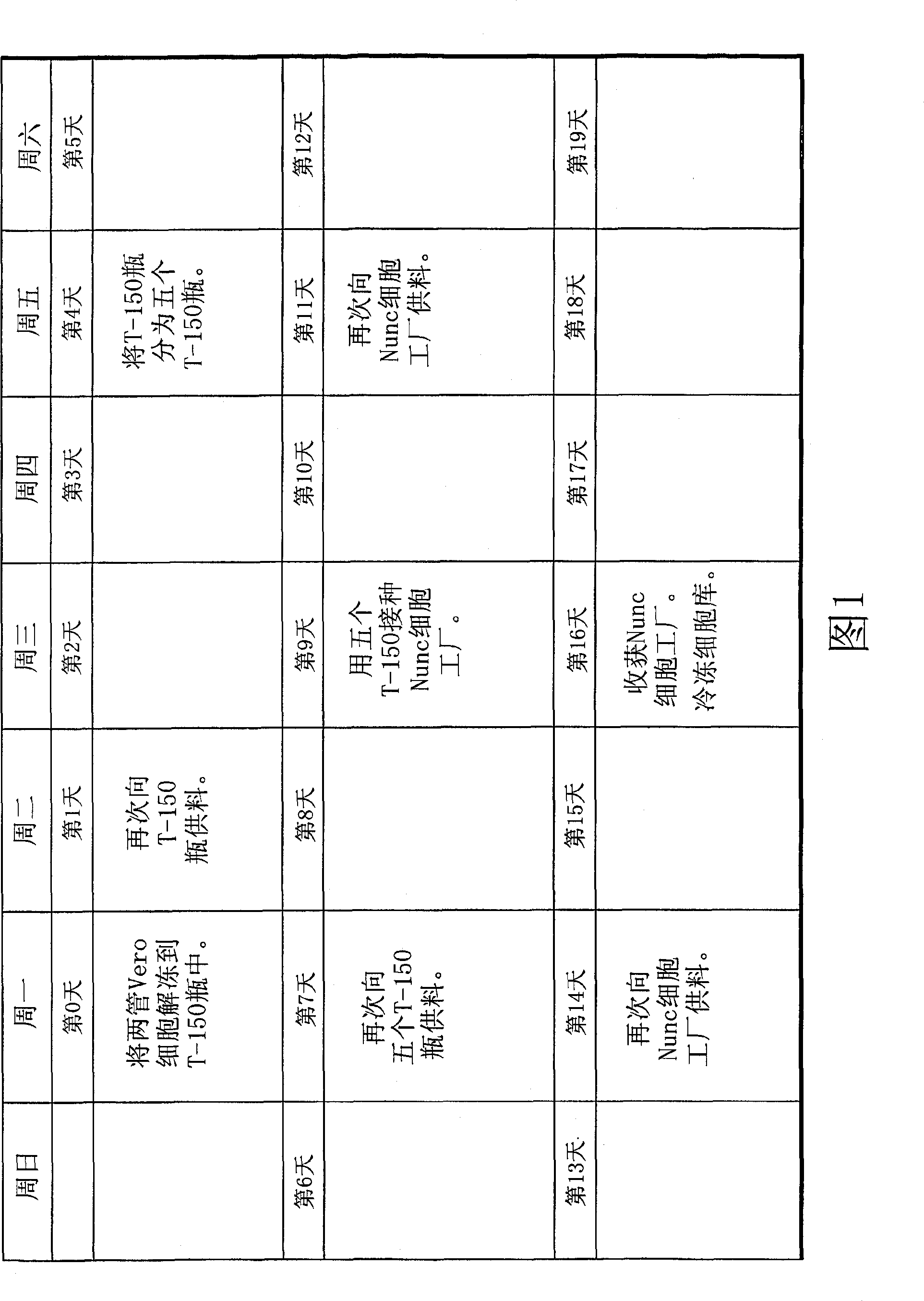
[0043] Thawing and Cell Growth
[0044] Put two containing 2×10 7 Frozen stock tubes of Vero cells were placed in a 37°C water bath. The tubes were kept in the water bath with agitation and checked frequently until the frozen cells were thawed. The outside of the tube was cleaned with 70% isopropanol and placed in a laminar flow hood. Quickly transfer thawed cells to a T-150cm 2 bottle and add 50 ml of growth medium dropwise while shaking the culture vessel. Growth medium consisted of VP-SFM with 4 mM L-glutamine. After about half of the medium was added, the remainder was added at a slightly faster rate. The cells were placed in a Forma incubator (37°C, 5% CO 2 ) and allowed to attach overnight. The medium was degassed and 50 ml of fresh growth medium was added to the bottle the next day.
example 2
[0046] cell delivery
[0047] Incubate cells in a Forma incubator at 37 °C with 5% CO 2 Under cultured T-150cm 2 Grow in bottles to confluency (4 or 5 days). The medium was vented and the bottle was washed twice with 20 ml PBS without magnesium and calcium. 5 ml of trypsin was added to the flask and the flask was incubated at room temperature for 2-3 minutes to remove cells from the flask. Trypsin was neutralized with 5 ml of STI and 10 ml of VP-SFM was added for nutritional support until the procedure was complete. The cells now in suspension were sampled for enumeration. Add 1 mL of trypan blue solution to 1 mL of the cell sample. Mix it gently with a vortex shaker. Place 10 µl of the mixture in the chamber of a hemocytometer. Cells that did not undergo staining were counted as viable and total cell numbers were determined.
[0048] The cell suspension was then divided into 4 × 10 4 The concentration of cells / cm2 was inoculated into five new T-150cm 2 in the bottle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com