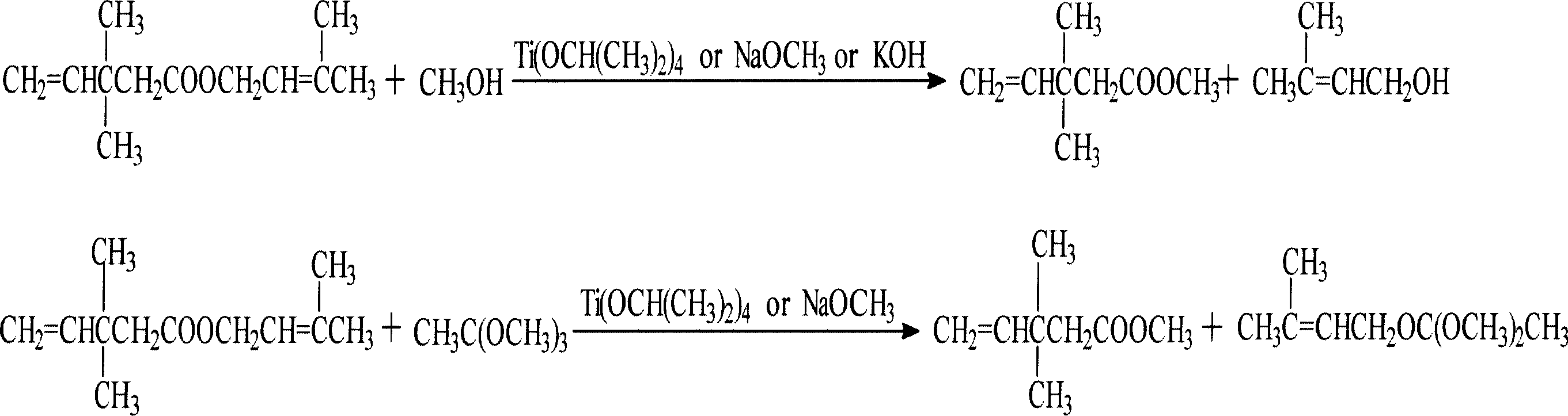Penisopenteneester recovery method
A technology of isopentenyl bentinate and a recovery method, which is applied in the fields of chemistry and pesticides, can solve problems such as high recovery cost, complex recovery process, and deteriorating working environment, and achieves reduction in production cost, simple recovery process, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
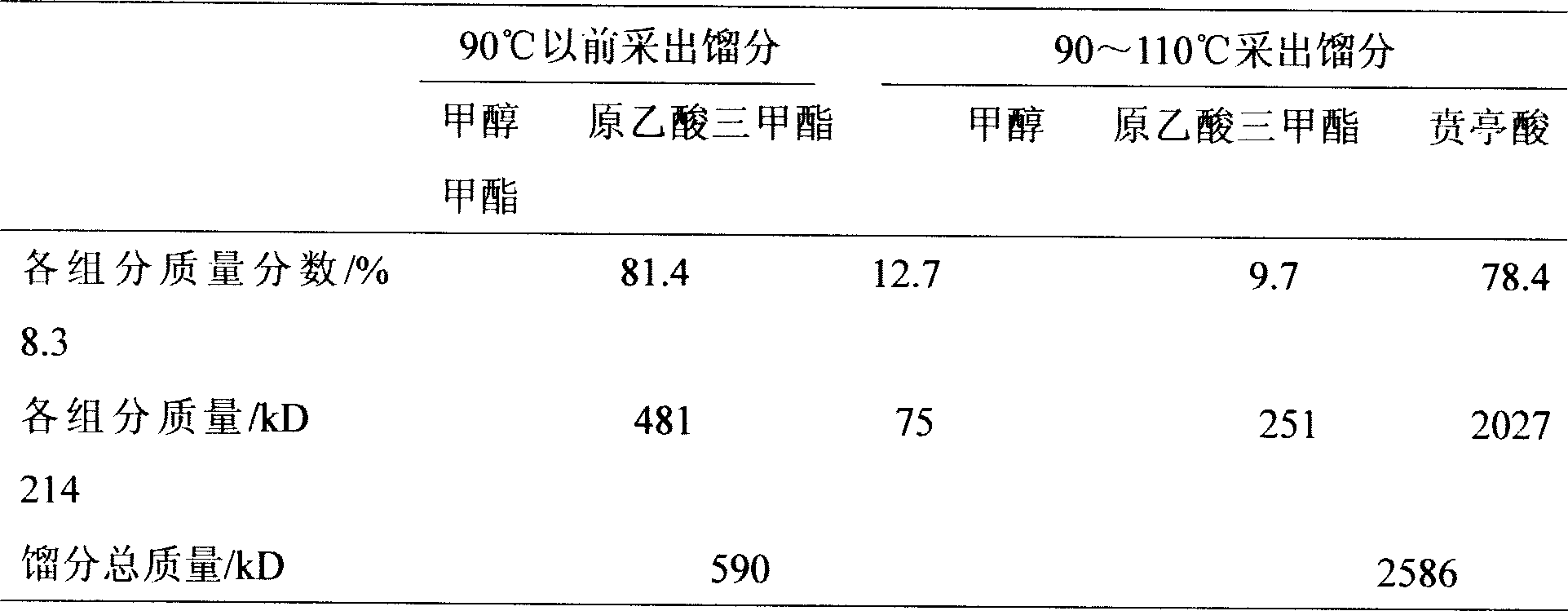
Examples
Embodiment 1
[0021] Embodiment 1: tetraisopropyl titanate as transesterification catalyst (A process)
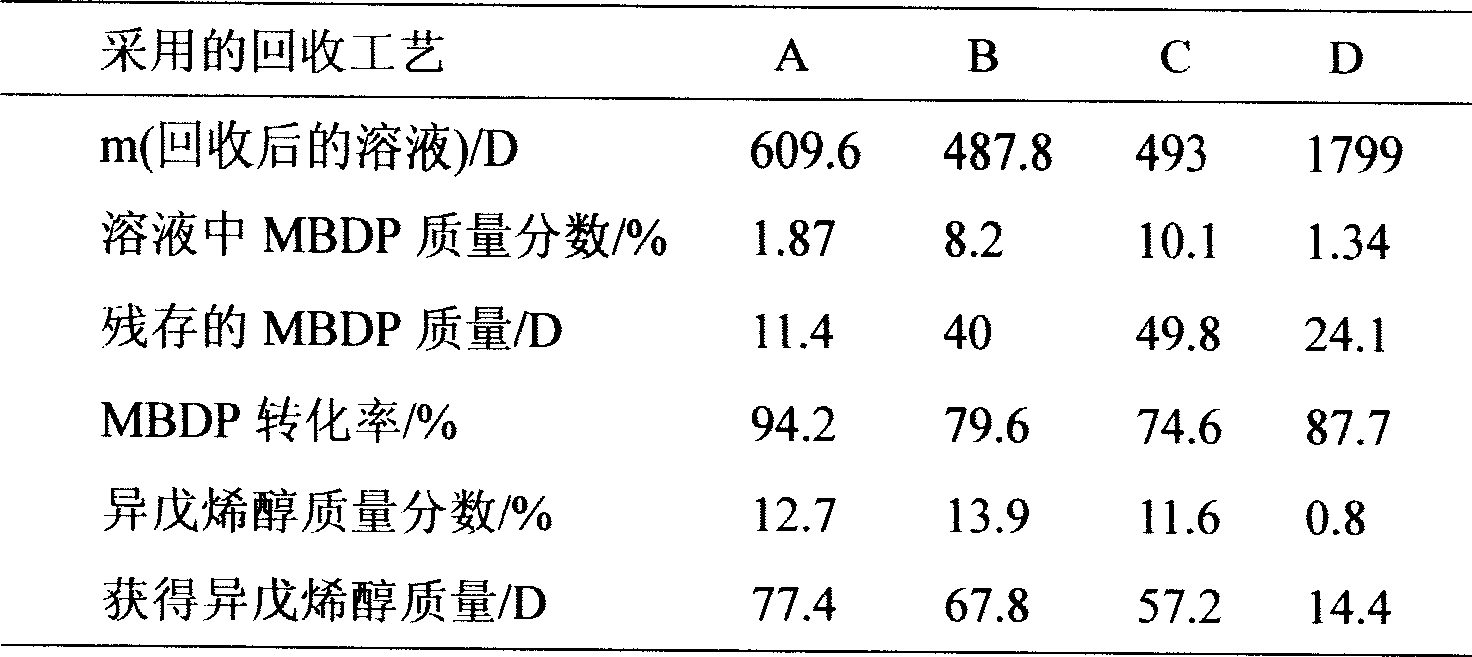
[0022] Add 312g of isopentenyl perineate (containing 1.0mol of MBDP), 320g of anhydrous methanol (10.0mol), and 3mL of tetraisopropyl titanate (0.01mol) into the DSH-2 high-pressure reactor, and start stirring , after the temperature was raised to 152°C, gas chromatographic analysis was taken every half hour. When the reaction time reached 7.5 hours, the mass fraction of MBDP tended to be stable, and the reaction was terminated. The reaction solution was cooled to room temperature, and DC analysis showed that the mass fraction of MBDP was 1.87 %, the residual mass of MBDP was 11.4g, and the conversion rate was 94.2%; the mass fraction and mass of prenol produced were 12.7% and 77.4g, respectively.
Embodiment 2
[0023] Embodiment 2: Sodium methylate is as transesterification catalyst (B technology)
[0024] Add isopentenyl perineate 312D (MBDP1.0mol), anhydrous methanol 192D (6.0mol), and liquid sodium methoxide 3.8g (0.02mol) into the high-pressure reactor, and after heating up to 140°C, gas chromatography tracking analysis , after the reaction time reaches 6h, the mass fraction of MBDP tends to be stable, the cooling reaction solution, DC analysis, the mass fraction of MBDP is 8.2%, the remaining MBDP mass is 40g, and the conversion rate is 79.6%; the mass fraction of prenol and The masses are 13.9%, 67.8g, respectively.
Embodiment 3
[0025] Embodiment 3: Potassium hydroxide is as transesterification catalyst (C technology)
[0026] Add respectively 312g (MBDP1.0mol) of isopentenyl perineate, 192g (6.0mol) of anhydrous methanol, 9.3g (0.02mol) of potassium hydroxide methanol solution of 12% in the autoclave, be warming up to 140 ℃, Follow-up analysis showed that after 9 hours of reaction, the reaction was terminated, the mass fraction of MBDP was 10.1%, the mass fraction of residual MBDP was 49.8g, and the conversion rate was 74.6%; the mass fraction and mass of prenol produced were 11.6% and 57.2g, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com