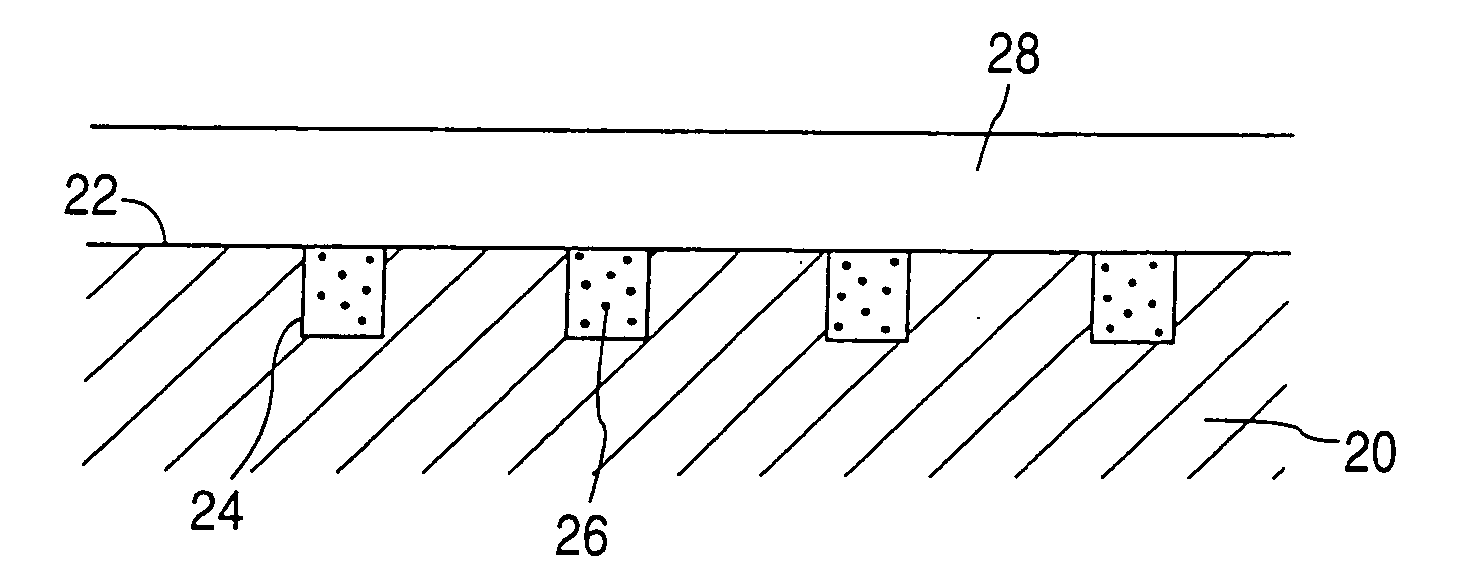
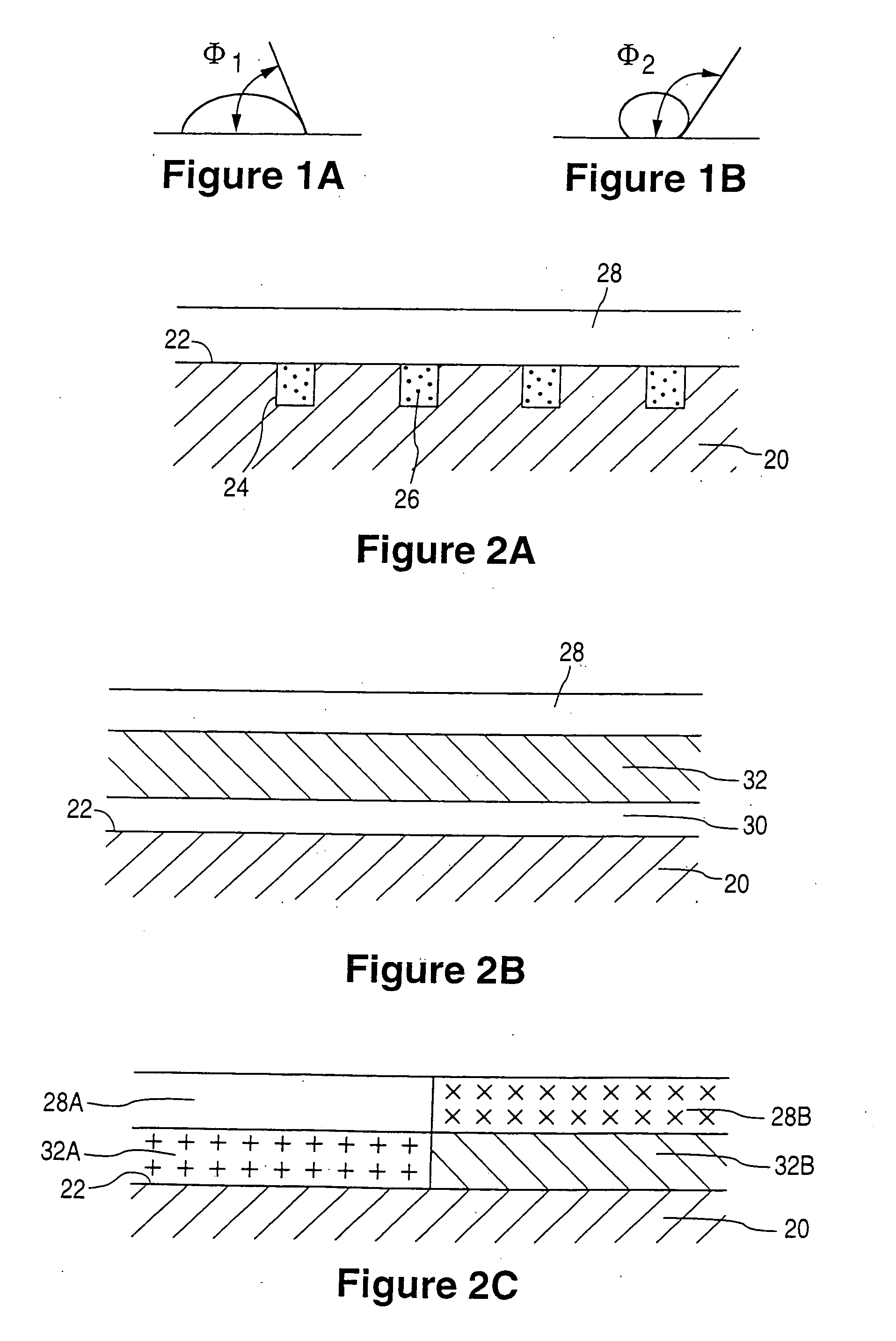
Rate-reducing membrane for release of an agent
a rate-reducing membrane and agent technology, applied in the coating field, can solve the problems of affecting the release rate of the agent, affecting the quality of the product, so as to reduce the rate of release of the agen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0078] A 13 mm, 316L stainless steel TETRA stent is primer coated by spraying with a 2% (w / w) solution of poly(ethylene-co-vinyl alcohol) (44 mole % ethylene) in dimethylacetamide. The solvent is removed by baking at 140° C. for 1 hour. A solution of 2% (w / w) EVAL and 0.25% (w / w) actinomycin D in dimethylacetamide is spray coated onto the stent to a thickness that gives 25 μg of actinomycin D on the stent. The stent is then baked at 50° C. for two hours. A hydrophobic release rate limiting membrane is formed by spraying the stent with a 2% (w / w) solution of polybutylmethacrylate in a 1 / 3 (w / w) mixture of ethyl acetate and cyclohexanone. A second two hour bake at 50° C. is performed to remove the solvent.
example 2
[0079] A 13 mm, 316L stainless steel TETRA stent is primer coated by spraying with a 2% (w / w) solution of poly(ethylene-co-vinyl alcohol) (44 mole % ethylene) in dimethylacetamide. The solvent is removed by baking at 140° C. for 1 hour. A solution of 2% (w / w) EVAL and 0.5% (w / w) paclitaxel in dimethylacetamide is spray coated onto the stent to a thickness that gives 50 μg of paclitaxel on the stent. The stent is then baked at 50° C. for two hours. A hydrophobic release rate limiting membrane is formed by spraying on a 2% (w / w) solution of poly(ethylene-co-vinylacetate) (25 mole % acetate content) in a 1 / 1 (w / w) solution of toluene and n-butyl acetate. Another two hour bake at 50° C. is performed to remove the solvent.
example 3
[0080] A 13 mm, 316L stainless steel TETRA stent is primer coated by spraying with a 2% (w / w) solution of poly(ethylene-co-vinyl alcohol) (44 mole % ethylene) in dimethylacetamide. The solvent is removed by baking at 140° C. for 1 hour. A solution of 2% (w / w) EVAL and 0.67% (w / w) clobetasol propionate in dimethylacetamide is spray coated onto the stent to a thickness that gives 150 μg of clobetasol propionate on the stent. The stent is then baked at 50° C. for two hours. A hydrophobic release rate limiting membrane is formed by coating on a 5% (w / w) solution of Nusil MED3-6605 silicone dispersion in a 1 / 1 (w / w) of trichloroethylene and cyclohexane. This process is accomplished by placing the stent on a section of 0.070 inch OD stainless steel tubing. The coating is then applied to the stent via syringe. With the stent covered with fluid, it is pushed along the length of the tube with a short section of TEFLON tubing, while simultaneously rotating the stainless steel tube. The coatin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole percentage | aaaaa | aaaaa |
| mole percent | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com