Electroless platinum plating solution
a technology of electroless platinum and plating solution, applied in the direction of liquid/solution decomposition chemical coating, metal material coating process, coating, etc., to achieve the effect of easy dissolved in water, easy reduction, and easy reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0050][Preparation of Plating Substrate]
[0051]First, a nickel film having a thickness of 3 μm was formed on a copper plate by electrolytic plating processing. Then, a plating substrate was prepared by forming a platinum film (electrolytic platinum film) having a thickness of 0.1 μm on the surface of the nickel film by electrolytic plating processing.
[0052][Preparation of Electroless Platinum Plating Solution]
[0053]In this Example, 0.005 mol / L of dichlorotetraammineplatinum (II) (1.0 g / L in terms of platinum) as a water-soluble platinum compound, 0.5 mol / L of sodium formate as a reducing agent, and 0.1 mol / L of malic acid as an complexing agent were dissolved in water to prepare an electroless platinum plating solution. Next, the pH (at a temperature of 25° C.) of the electroless platinum plating solution was adjusted to 7.0 by using sodium hydroxide and sulfuric acid as pH adjusting agents.
[0054][Electroless Plating Processing]
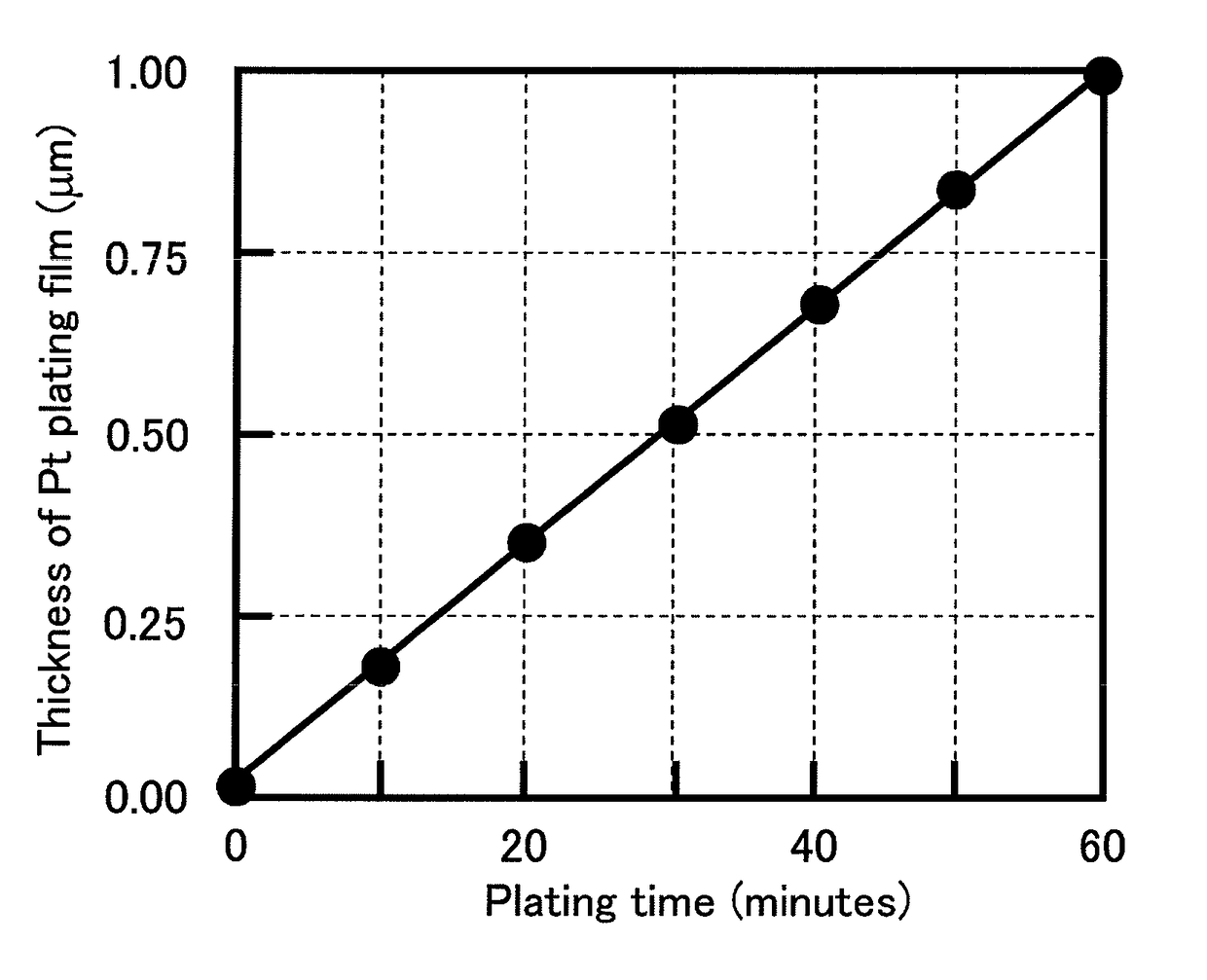
[0055]The resulting electroless platinum plating solutio...
example 2
[0060]In this example, the electroless platinum plating solution was prepared in exactly the same manner as in Example 1 except that the pH (at a temperature of 25° C.) was adjusted to 11.0.
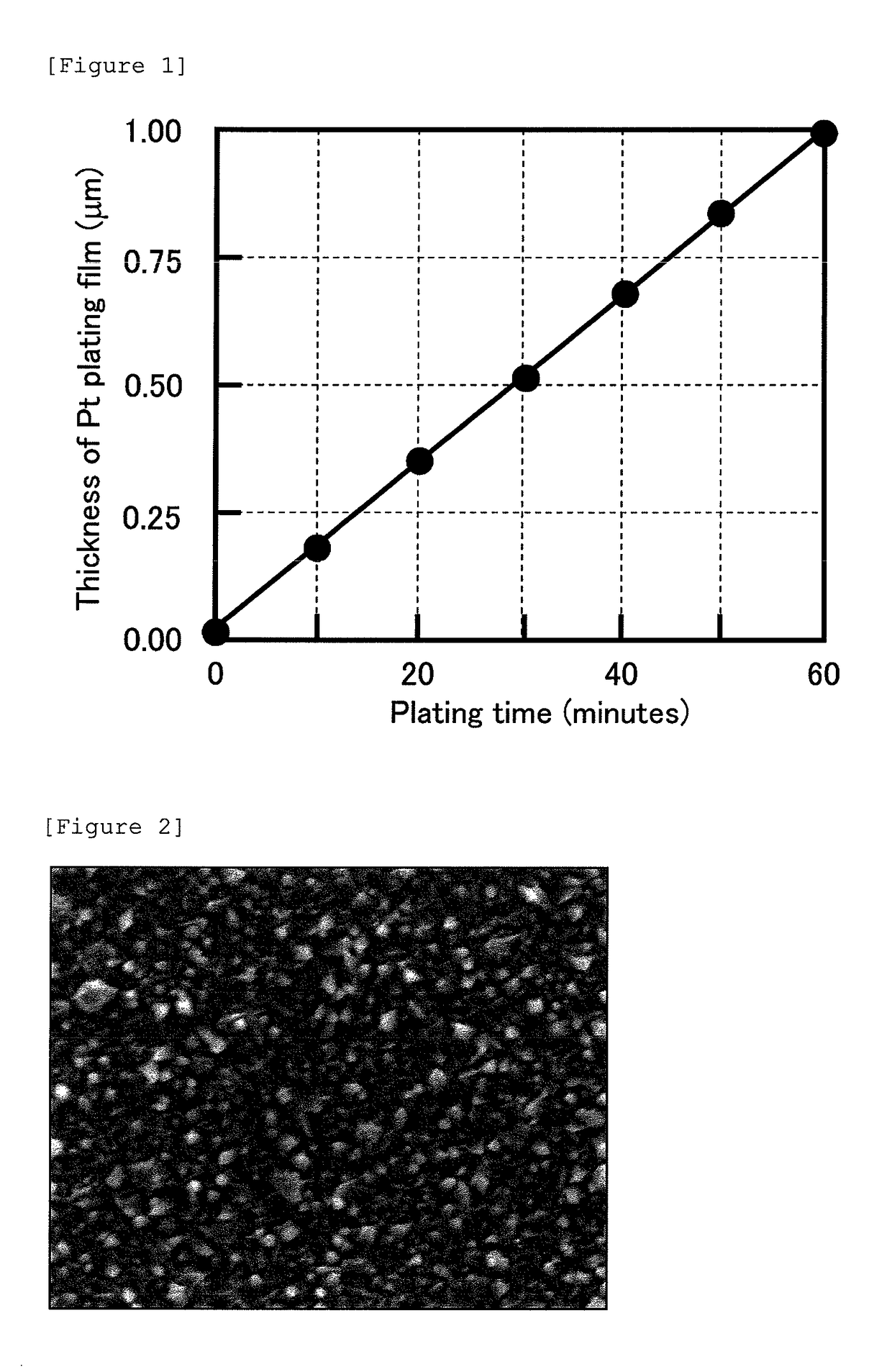
[0061]The resulting electroless platinum plating solution was warmed to a temperature of 70° C., the plating substrate having the electrolytic platinum film thereon was subjected to electroless plating processing by immersing it in the electroless platinum plating solution for 60 minutes to form an electroless platinum film having a thickness of 0.5 μm on the electrolytic platinum film. The resulting electroless platinum film was visually observed to have a white glossy appearance. In addition, the surface of the resulting electroless platinum film was observed with a SEM at a magnification of 30,000 times. As shown in FIG. 3, the particle size of the platinum particles was small, and the electroless platinum film has a smooth surface shape.
[0062]Then, the resulting electroless platinum plating s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| deposition rate | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com