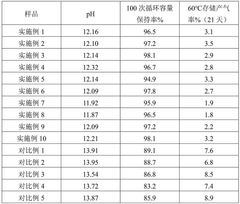
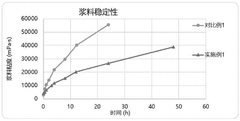
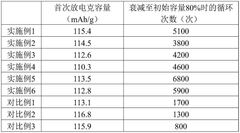
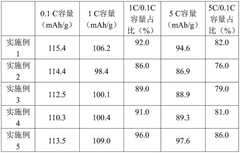
Advanced Characterization Techniques in Sodium Ion Battery Research
AUG 7, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-Ion Battery Research Background and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries (LIBs) in recent years, driven by the increasing demand for sustainable and cost-effective energy storage solutions. The development of SIBs can be traced back to the 1980s, but significant progress has been made in the past decade due to advancements in materials science and characterization techniques.
The primary objective of sodium-ion battery research is to develop high-performance, safe, and economically viable energy storage systems that can complement or potentially replace LIBs in certain applications. This goal is motivated by the abundance and wide geographical distribution of sodium resources, which could lead to reduced production costs and improved supply chain stability.
Advanced characterization techniques play a crucial role in understanding the fundamental mechanisms and processes occurring within sodium-ion batteries. These techniques enable researchers to investigate the structural, chemical, and electrochemical properties of battery materials at various length scales and time resolutions. By providing detailed insights into the behavior of electrode materials, electrolytes, and interfaces, these characterization methods contribute to the optimization of battery performance and the development of novel materials.
The evolution of characterization techniques in SIB research has been closely linked to the progress in analytical instrumentation and computational capabilities. From conventional electrochemical methods to advanced spectroscopic and microscopic techniques, the field has witnessed a significant expansion in the tools available for battery characterization. This has led to a more comprehensive understanding of the complex phenomena occurring during battery operation, including ion insertion/extraction mechanisms, structural changes, and degradation processes.
As the field of sodium-ion battery research continues to advance, there is a growing emphasis on in situ and operando characterization techniques. These methods allow for real-time observation of battery processes under realistic operating conditions, providing valuable information that cannot be obtained through ex situ measurements alone. The integration of multiple characterization techniques and the development of synchrotron-based methods have further enhanced our ability to probe battery materials and systems with unprecedented detail and accuracy.
Looking ahead, the future of sodium-ion battery research is expected to focus on addressing key challenges such as improving energy density, enhancing cycling stability, and developing new electrode materials and electrolytes. Advanced characterization techniques will continue to play a vital role in these efforts, enabling researchers to unravel complex mechanisms, identify performance limitations, and guide the rational design of next-generation sodium-ion batteries.
The primary objective of sodium-ion battery research is to develop high-performance, safe, and economically viable energy storage systems that can complement or potentially replace LIBs in certain applications. This goal is motivated by the abundance and wide geographical distribution of sodium resources, which could lead to reduced production costs and improved supply chain stability.
Advanced characterization techniques play a crucial role in understanding the fundamental mechanisms and processes occurring within sodium-ion batteries. These techniques enable researchers to investigate the structural, chemical, and electrochemical properties of battery materials at various length scales and time resolutions. By providing detailed insights into the behavior of electrode materials, electrolytes, and interfaces, these characterization methods contribute to the optimization of battery performance and the development of novel materials.
The evolution of characterization techniques in SIB research has been closely linked to the progress in analytical instrumentation and computational capabilities. From conventional electrochemical methods to advanced spectroscopic and microscopic techniques, the field has witnessed a significant expansion in the tools available for battery characterization. This has led to a more comprehensive understanding of the complex phenomena occurring during battery operation, including ion insertion/extraction mechanisms, structural changes, and degradation processes.
As the field of sodium-ion battery research continues to advance, there is a growing emphasis on in situ and operando characterization techniques. These methods allow for real-time observation of battery processes under realistic operating conditions, providing valuable information that cannot be obtained through ex situ measurements alone. The integration of multiple characterization techniques and the development of synchrotron-based methods have further enhanced our ability to probe battery materials and systems with unprecedented detail and accuracy.
Looking ahead, the future of sodium-ion battery research is expected to focus on addressing key challenges such as improving energy density, enhancing cycling stability, and developing new electrode materials and electrolytes. Advanced characterization techniques will continue to play a vital role in these efforts, enabling researchers to unravel complex mechanisms, identify performance limitations, and guide the rational design of next-generation sodium-ion batteries.
Market Analysis for Na-Ion Battery Technology
The sodium-ion battery market is experiencing rapid growth and attracting significant attention from both industry and academia. This emerging technology is positioned as a potential alternative to lithium-ion batteries, particularly in large-scale energy storage applications and electric vehicles. The market demand for sodium-ion batteries is driven by several factors, including the abundance and low cost of sodium resources, the potential for improved safety, and the need for sustainable energy storage solutions.
Current market projections indicate a compound annual growth rate (CAGR) of over 20% for the sodium-ion battery market in the coming years. This growth is fueled by increasing investments in research and development, as well as pilot production projects by major battery manufacturers and automotive companies. The market size is expected to reach several billion dollars by 2030, with Asia-Pacific region, particularly China, leading in terms of production capacity and technological advancements.
The demand for sodium-ion batteries is particularly strong in the renewable energy sector, where large-scale energy storage systems are crucial for grid stability and integration of intermittent power sources. Additionally, the electric vehicle market is showing interest in sodium-ion technology as a potential solution for low-cost, long-range vehicles, especially in emerging markets where cost sensitivity is high.
Key market segments for sodium-ion batteries include stationary energy storage, electric vehicles, and consumer electronics. In the stationary storage sector, sodium-ion batteries are being considered for grid-level applications, renewable energy integration, and backup power systems. For electric vehicles, the focus is on developing high-energy-density sodium-ion cells that can compete with lithium-ion batteries in terms of range and performance.
The market landscape is characterized by a mix of established battery manufacturers expanding into sodium-ion technology and startups specializing in this field. Major players are investing in pilot production lines and collaborating with research institutions to accelerate commercialization. Government initiatives and funding programs in various countries are also contributing to market growth by supporting research and development efforts.
Despite the promising outlook, challenges remain in scaling up production and achieving cost parity with lithium-ion batteries. The success of sodium-ion technology in capturing market share will depend on continued improvements in energy density, cycle life, and manufacturing processes. As advanced characterization techniques play a crucial role in optimizing battery performance and reliability, their development is closely tied to the overall market potential of sodium-ion batteries.
Current market projections indicate a compound annual growth rate (CAGR) of over 20% for the sodium-ion battery market in the coming years. This growth is fueled by increasing investments in research and development, as well as pilot production projects by major battery manufacturers and automotive companies. The market size is expected to reach several billion dollars by 2030, with Asia-Pacific region, particularly China, leading in terms of production capacity and technological advancements.
The demand for sodium-ion batteries is particularly strong in the renewable energy sector, where large-scale energy storage systems are crucial for grid stability and integration of intermittent power sources. Additionally, the electric vehicle market is showing interest in sodium-ion technology as a potential solution for low-cost, long-range vehicles, especially in emerging markets where cost sensitivity is high.
Key market segments for sodium-ion batteries include stationary energy storage, electric vehicles, and consumer electronics. In the stationary storage sector, sodium-ion batteries are being considered for grid-level applications, renewable energy integration, and backup power systems. For electric vehicles, the focus is on developing high-energy-density sodium-ion cells that can compete with lithium-ion batteries in terms of range and performance.
The market landscape is characterized by a mix of established battery manufacturers expanding into sodium-ion technology and startups specializing in this field. Major players are investing in pilot production lines and collaborating with research institutions to accelerate commercialization. Government initiatives and funding programs in various countries are also contributing to market growth by supporting research and development efforts.
Despite the promising outlook, challenges remain in scaling up production and achieving cost parity with lithium-ion batteries. The success of sodium-ion technology in capturing market share will depend on continued improvements in energy density, cycle life, and manufacturing processes. As advanced characterization techniques play a crucial role in optimizing battery performance and reliability, their development is closely tied to the overall market potential of sodium-ion batteries.
Current Challenges in Na-Ion Battery Characterization
Despite significant advancements in sodium-ion battery research, several challenges persist in the characterization of these systems. One of the primary obstacles is the limited availability of specialized characterization techniques tailored specifically for sodium-ion batteries. Many existing methods have been adapted from lithium-ion battery research, which may not fully capture the unique properties and behaviors of sodium-based systems.
The high reactivity of sodium with air and moisture poses significant challenges for in situ and operando characterization techniques. This reactivity necessitates stringent environmental controls during analysis, often complicating experimental setups and potentially introducing artifacts in the data. Additionally, the larger ionic radius of sodium compared to lithium can lead to more complex structural changes during cycling, making it difficult to accurately track and interpret these transformations using conventional characterization methods.
Another critical challenge lies in the development of suitable reference electrodes for sodium-ion systems. The lack of a universally accepted reference electrode complicates the comparison of results across different studies and hinders the standardization of characterization protocols. This issue is particularly pronounced in three-electrode cell configurations, which are crucial for isolating and studying individual electrode behaviors.
The characterization of solid electrolyte interphase (SEI) formation and evolution in sodium-ion batteries presents unique challenges. The SEI in these systems often exhibits different composition and stability compared to lithium-ion batteries, necessitating the development of specialized techniques to probe its structure and properties accurately. Furthermore, the dynamic nature of the SEI during cycling adds another layer of complexity to its characterization.
Quantitative analysis of sodium distribution and transport within electrode materials remains a significant challenge. While techniques such as neutron diffraction and nuclear magnetic resonance (NMR) spectroscopy have shown promise, they often require specialized facilities and expertise, limiting their widespread adoption. The development of more accessible and high-resolution techniques for mapping sodium distribution is crucial for optimizing electrode designs and understanding degradation mechanisms.
Lastly, the characterization of emerging electrode materials and electrolytes for sodium-ion batteries poses unique challenges. Many of these novel materials exhibit complex structures and chemistries that are not easily probed by conventional techniques. This necessitates the development of new characterization methodologies and the adaptation of existing ones to accurately assess their properties and performance in sodium-ion systems.
The high reactivity of sodium with air and moisture poses significant challenges for in situ and operando characterization techniques. This reactivity necessitates stringent environmental controls during analysis, often complicating experimental setups and potentially introducing artifacts in the data. Additionally, the larger ionic radius of sodium compared to lithium can lead to more complex structural changes during cycling, making it difficult to accurately track and interpret these transformations using conventional characterization methods.
Another critical challenge lies in the development of suitable reference electrodes for sodium-ion systems. The lack of a universally accepted reference electrode complicates the comparison of results across different studies and hinders the standardization of characterization protocols. This issue is particularly pronounced in three-electrode cell configurations, which are crucial for isolating and studying individual electrode behaviors.
The characterization of solid electrolyte interphase (SEI) formation and evolution in sodium-ion batteries presents unique challenges. The SEI in these systems often exhibits different composition and stability compared to lithium-ion batteries, necessitating the development of specialized techniques to probe its structure and properties accurately. Furthermore, the dynamic nature of the SEI during cycling adds another layer of complexity to its characterization.
Quantitative analysis of sodium distribution and transport within electrode materials remains a significant challenge. While techniques such as neutron diffraction and nuclear magnetic resonance (NMR) spectroscopy have shown promise, they often require specialized facilities and expertise, limiting their widespread adoption. The development of more accessible and high-resolution techniques for mapping sodium distribution is crucial for optimizing electrode designs and understanding degradation mechanisms.
Lastly, the characterization of emerging electrode materials and electrolytes for sodium-ion batteries poses unique challenges. Many of these novel materials exhibit complex structures and chemistries that are not easily probed by conventional techniques. This necessitates the development of new characterization methodologies and the adaptation of existing ones to accurately assess their properties and performance in sodium-ion systems.
State-of-the-Art Characterization Methods for Na-Ion Batteries
01 Electrochemical characterization techniques
Various electrochemical techniques are used to characterize sodium-ion batteries, including cyclic voltammetry, galvanostatic charge-discharge, and electrochemical impedance spectroscopy. These methods help evaluate battery performance, cycling stability, and internal resistance, providing crucial insights into the electrochemical behavior of sodium-ion battery components.- Electrochemical characterization techniques: Various electrochemical techniques are employed to characterize sodium-ion batteries, including cyclic voltammetry, galvanostatic charge-discharge tests, and electrochemical impedance spectroscopy. These methods help evaluate battery performance, cycling stability, and internal resistance, providing crucial insights into the electrochemical behavior of sodium-ion battery components.
- Structural and morphological analysis: Advanced microscopy and spectroscopy techniques are used to analyze the structural and morphological properties of sodium-ion battery materials. These include scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Raman spectroscopy. These methods provide valuable information about particle size, crystal structure, and surface morphology of electrode materials and electrolytes.
- In-situ and operando characterization methods: In-situ and operando characterization techniques allow for real-time monitoring of sodium-ion battery processes during operation. These methods include in-situ XRD, in-situ Raman spectroscopy, and operando neutron diffraction. They provide insights into structural changes, ion transport mechanisms, and interfacial reactions occurring within the battery during charge-discharge cycles.
- Thermal analysis and safety evaluation: Thermal characterization techniques are crucial for assessing the safety and stability of sodium-ion batteries. Methods such as differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and accelerated rate calorimetry (ARC) are used to study thermal behavior, decomposition temperatures, and potential safety hazards associated with battery materials and components.
- Computational modeling and simulation: Computational methods, including density functional theory (DFT) calculations and molecular dynamics simulations, are employed to predict and analyze the properties of sodium-ion battery materials. These techniques help in understanding ion diffusion mechanisms, electronic structures, and interfacial phenomena, guiding the design and optimization of battery components.
02 Structural and morphological analysis
Advanced microscopy and spectroscopy techniques are employed to analyze the structural and morphological characteristics of sodium-ion battery materials. These include scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Raman spectroscopy, which provide valuable information about material composition, crystal structure, and surface properties.Expand Specific Solutions03 In-situ and operando characterization methods
Real-time characterization techniques are utilized to study sodium-ion batteries during operation. These methods include in-situ XRD, operando neutron diffraction, and synchrotron-based X-ray absorption spectroscopy, allowing researchers to observe structural and chemical changes in battery materials during charge-discharge cycles and providing insights into degradation mechanisms.Expand Specific Solutions04 Thermal analysis and safety evaluation
Thermal characterization techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are used to assess the thermal stability and safety of sodium-ion battery components. These methods help identify potential thermal runaway conditions and evaluate the effectiveness of safety measures implemented in battery design.Expand Specific Solutions05 Computational modeling and simulation
Advanced computational techniques, including density functional theory (DFT) calculations and molecular dynamics simulations, are employed to predict and analyze the behavior of sodium-ion battery materials. These methods aid in understanding ion transport mechanisms, predicting material properties, and optimizing battery designs for improved performance and longevity.Expand Specific Solutions
Key Players in Na-Ion Battery Research and Development
The advanced characterization techniques in sodium ion battery research field is currently in a growth phase, with increasing market size and technological advancements. The global market for sodium ion batteries is expanding, driven by the demand for sustainable energy storage solutions. Technologically, the field is progressing rapidly, with companies like Faradion Ltd. and StoreDot Ltd. leading innovations in sodium ion battery materials and extreme fast charging technologies. Established players such as 3M Innovative Properties Co. and Sharp Corp. are also contributing to the development of advanced characterization techniques. Research institutions like Centre National de la Recherche Scientifique and Sorbonne Université are playing crucial roles in advancing fundamental understanding. The collaboration between industry and academia is accelerating the maturation of these technologies, positioning sodium ion batteries as a promising alternative to lithium ion batteries.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced characterization techniques for sodium-ion batteries, focusing on operando synchrotron X-ray diffraction and X-ray absorption spectroscopy. These methods allow for real-time monitoring of structural and electronic changes in electrode materials during battery operation[1]. They have also implemented in situ NMR spectroscopy to study the local environment of sodium ions in the electrolyte and at the electrode-electrolyte interface[2]. Additionally, CNRS researchers have utilized advanced electron microscopy techniques, including in situ TEM, to observe the formation and evolution of the solid electrolyte interphase (SEI) in sodium-ion batteries[3].
Strengths: Access to state-of-the-art synchrotron facilities and expertise in operando characterization techniques. Weaknesses: May face challenges in translating fundamental research findings into practical battery designs for commercial applications.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: CEA has developed a suite of advanced characterization techniques for sodium-ion battery research, including high-resolution transmission electron microscopy (HRTEM) coupled with electron energy loss spectroscopy (EELS) to study the local structure and chemistry of electrode materials at the atomic scale[4]. They have also implemented in situ X-ray photoelectron spectroscopy (XPS) to investigate the surface chemistry of electrodes and the formation of solid electrolyte interphase (SEI) layers[5]. Furthermore, CEA researchers have utilized neutron diffraction techniques to probe the sodium insertion/extraction mechanisms in various electrode materials, providing insights into the structural changes during battery cycling[6].
Strengths: Comprehensive suite of characterization techniques covering multiple length scales and phenomena. Weaknesses: May face challenges in integrating multiple characterization techniques for a holistic understanding of battery performance.
Innovative Characterization Approaches for Na-Ion Systems
Sodium ion battery positive electrode material, preparation method therefor, positive electrode and sodium ion battery
PatentWO2025002026A1
Innovation
- By coating tungsten-containing compounds and doping effective elements such as Al, Mg, Ti, Zr, Y, La, Cu, Zn, Ca, Li or B, the residual alkali amount on the surface of the positive electrode material is reduced and the structural stability of the material is improved. and ionic conductivity.
Sodium-ion battery positive electrode material and preparation method therefor, and sodium-ion battery
PatentWO2024250412A1
Innovation
- By introducing doping of magnesium ions and M ions into the positive electrode material of the sodium ion battery and coating of an alumina coating film, the MgxFeyMnzM(1-x-y-z)(OH)2 core and Al(OH)3 coated film structure are formed. Improve the structural stability and electrochemical properties of the material.
Environmental Impact of Na-Ion Battery Technology
The environmental impact of Na-ion battery technology is a crucial consideration as this emerging energy storage solution gains traction. Compared to traditional lithium-ion batteries, sodium-ion batteries offer several environmental advantages. Firstly, sodium is significantly more abundant and widely distributed than lithium, reducing the environmental strain associated with resource extraction. This abundance translates to lower mining impacts and reduced geopolitical tensions over resource control.
In terms of production, Na-ion batteries generally require less energy-intensive manufacturing processes. The lower operating temperatures and simpler electrode materials contribute to a reduced carbon footprint during production. Additionally, the use of aluminum instead of copper for the current collector in the anode further decreases the environmental burden and cost of production.
The life cycle assessment of Na-ion batteries reveals potential benefits in terms of greenhouse gas emissions and energy consumption. Studies have shown that the global warming potential of Na-ion batteries can be up to 20% lower than that of lithium-ion batteries, primarily due to the differences in raw material extraction and processing.
However, it is important to note that the environmental impact of Na-ion batteries is not entirely benign. The production of sodium carbonate, a key component in Na-ion batteries, still requires significant energy input. Moreover, the extraction of other materials used in these batteries, such as hard carbon for anodes, may have localized environmental impacts that need to be carefully managed.
End-of-life considerations for Na-ion batteries are generally favorable. The materials used are typically less toxic and more easily recyclable than those in lithium-ion batteries. This characteristic facilitates more efficient and environmentally friendly recycling processes, potentially leading to a more circular economy in battery production and disposal.
As Na-ion battery technology continues to evolve, ongoing research is focused on further improving its environmental profile. This includes developing more sustainable cathode materials, enhancing energy density to reduce material requirements, and optimizing recycling techniques. The integration of Na-ion batteries into renewable energy systems also holds promise for reducing overall environmental impact in the energy sector.
In terms of production, Na-ion batteries generally require less energy-intensive manufacturing processes. The lower operating temperatures and simpler electrode materials contribute to a reduced carbon footprint during production. Additionally, the use of aluminum instead of copper for the current collector in the anode further decreases the environmental burden and cost of production.
The life cycle assessment of Na-ion batteries reveals potential benefits in terms of greenhouse gas emissions and energy consumption. Studies have shown that the global warming potential of Na-ion batteries can be up to 20% lower than that of lithium-ion batteries, primarily due to the differences in raw material extraction and processing.
However, it is important to note that the environmental impact of Na-ion batteries is not entirely benign. The production of sodium carbonate, a key component in Na-ion batteries, still requires significant energy input. Moreover, the extraction of other materials used in these batteries, such as hard carbon for anodes, may have localized environmental impacts that need to be carefully managed.
End-of-life considerations for Na-ion batteries are generally favorable. The materials used are typically less toxic and more easily recyclable than those in lithium-ion batteries. This characteristic facilitates more efficient and environmentally friendly recycling processes, potentially leading to a more circular economy in battery production and disposal.
As Na-ion battery technology continues to evolve, ongoing research is focused on further improving its environmental profile. This includes developing more sustainable cathode materials, enhancing energy density to reduce material requirements, and optimizing recycling techniques. The integration of Na-ion batteries into renewable energy systems also holds promise for reducing overall environmental impact in the energy sector.
Standardization Efforts in Na-Ion Battery Characterization
Standardization efforts in Na-ion battery characterization have become increasingly important as the technology advances towards commercialization. These efforts aim to establish consistent protocols and methodologies for evaluating the performance, safety, and reliability of sodium-ion batteries across different research groups and industries.
One of the primary focuses of standardization is the development of uniform testing procedures for key battery parameters. This includes standardized methods for measuring capacity, cycling stability, rate capability, and coulombic efficiency. By adopting these standardized tests, researchers and manufacturers can ensure that their results are comparable and reproducible, facilitating more effective collaboration and accelerating the overall progress in the field.
Efforts are also underway to standardize the reporting of battery composition and materials. This involves establishing guidelines for describing electrode materials, electrolyte compositions, and cell configurations. Standardized nomenclature and reporting formats help in reducing ambiguity and improving the clarity of scientific communications in the Na-ion battery research community.
Safety characterization is another critical area where standardization is being pursued. Developing uniform protocols for assessing thermal stability, abuse tolerance, and failure modes of Na-ion batteries is essential for ensuring their safe implementation in various applications. These standardized safety tests are crucial for building consumer confidence and meeting regulatory requirements as Na-ion batteries move towards widespread adoption.
Electrochemical characterization techniques are also being standardized to provide a more comprehensive understanding of Na-ion battery performance. This includes establishing protocols for techniques such as cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic intermittent titration technique (GITT). Standardizing these advanced characterization methods enables researchers to gain deeper insights into the fundamental processes occurring within Na-ion batteries.
International organizations and consortia are playing a vital role in driving these standardization efforts. Bodies such as the International Electrotechnical Commission (IEC) and various national standards organizations are working towards developing globally recognized standards for Na-ion battery characterization. These collaborative efforts involve input from academic researchers, industry experts, and regulatory bodies to ensure that the developed standards are comprehensive and widely applicable.
As Na-ion battery technology continues to mature, these standardization efforts will play a crucial role in facilitating its integration into various applications and markets. By establishing a common language and set of protocols for characterization, the Na-ion battery community can accelerate innovation, improve quality control, and enhance the overall reliability and performance of this promising energy storage technology.
One of the primary focuses of standardization is the development of uniform testing procedures for key battery parameters. This includes standardized methods for measuring capacity, cycling stability, rate capability, and coulombic efficiency. By adopting these standardized tests, researchers and manufacturers can ensure that their results are comparable and reproducible, facilitating more effective collaboration and accelerating the overall progress in the field.
Efforts are also underway to standardize the reporting of battery composition and materials. This involves establishing guidelines for describing electrode materials, electrolyte compositions, and cell configurations. Standardized nomenclature and reporting formats help in reducing ambiguity and improving the clarity of scientific communications in the Na-ion battery research community.
Safety characterization is another critical area where standardization is being pursued. Developing uniform protocols for assessing thermal stability, abuse tolerance, and failure modes of Na-ion batteries is essential for ensuring their safe implementation in various applications. These standardized safety tests are crucial for building consumer confidence and meeting regulatory requirements as Na-ion batteries move towards widespread adoption.
Electrochemical characterization techniques are also being standardized to provide a more comprehensive understanding of Na-ion battery performance. This includes establishing protocols for techniques such as cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic intermittent titration technique (GITT). Standardizing these advanced characterization methods enables researchers to gain deeper insights into the fundamental processes occurring within Na-ion batteries.
International organizations and consortia are playing a vital role in driving these standardization efforts. Bodies such as the International Electrotechnical Commission (IEC) and various national standards organizations are working towards developing globally recognized standards for Na-ion battery characterization. These collaborative efforts involve input from academic researchers, industry experts, and regulatory bodies to ensure that the developed standards are comprehensive and widely applicable.
As Na-ion battery technology continues to mature, these standardization efforts will play a crucial role in facilitating its integration into various applications and markets. By establishing a common language and set of protocols for characterization, the Na-ion battery community can accelerate innovation, improve quality control, and enhance the overall reliability and performance of this promising energy storage technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!