Ammonium Hydroxide in the Synthesis of Zeolites: Structural Determinants
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeolite Synthesis Background and Objectives
Zeolites have been a subject of intense research and development for over six decades, with their unique structural properties making them invaluable in various industrial applications. The synthesis of zeolites has evolved significantly since their first artificial production in the 1940s, with ammonium hydroxide playing a crucial role in shaping their structural characteristics.
The primary objective of this technical research is to explore the intricate relationship between ammonium hydroxide and the structural determinants of zeolites during synthesis. This investigation aims to uncover the mechanisms by which ammonium hydroxide influences the formation, crystallization, and final structure of zeolite frameworks.
Historically, zeolite synthesis has progressed from simple hydrothermal methods to more sophisticated techniques involving organic structure-directing agents. Ammonium hydroxide has emerged as a key component in many of these advanced synthesis routes, acting as both a mineralizer and a pH regulator.
The technological evolution in zeolite synthesis has been driven by the demand for materials with specific pore sizes, shapes, and distributions. These tailored properties are essential for applications ranging from catalysis and gas separation to ion exchange and water purification. Understanding the role of ammonium hydroxide in controlling these structural features is paramount to advancing zeolite technology.
Current research focuses on elucidating the precise mechanisms by which ammonium hydroxide affects zeolite nucleation, crystal growth, and framework stability. This includes investigating its impact on the formation of secondary building units (SBUs), the assembly of these units into larger structures, and the final topology of the zeolite framework.
The goal is to develop a comprehensive model that can predict and control zeolite structure based on the concentration and application method of ammonium hydroxide during synthesis. This model would enable the design of zeolites with unprecedented precision, opening new avenues for their application in emerging fields such as nanotechnology and renewable energy.
Furthermore, this research aims to explore the potential of ammonium hydroxide in the synthesis of novel zeolite structures that have not been achievable through conventional methods. By manipulating the structural determinants influenced by ammonium hydroxide, it may be possible to create zeolites with unique properties and functionalities.
Ultimately, this technical investigation seeks to bridge the gap between fundamental understanding and practical application, providing insights that can be translated into improved industrial processes for zeolite production. The findings are expected to contribute significantly to the ongoing efforts to expand the zeolite family and enhance their performance in existing and future applications.
The primary objective of this technical research is to explore the intricate relationship between ammonium hydroxide and the structural determinants of zeolites during synthesis. This investigation aims to uncover the mechanisms by which ammonium hydroxide influences the formation, crystallization, and final structure of zeolite frameworks.
Historically, zeolite synthesis has progressed from simple hydrothermal methods to more sophisticated techniques involving organic structure-directing agents. Ammonium hydroxide has emerged as a key component in many of these advanced synthesis routes, acting as both a mineralizer and a pH regulator.
The technological evolution in zeolite synthesis has been driven by the demand for materials with specific pore sizes, shapes, and distributions. These tailored properties are essential for applications ranging from catalysis and gas separation to ion exchange and water purification. Understanding the role of ammonium hydroxide in controlling these structural features is paramount to advancing zeolite technology.
Current research focuses on elucidating the precise mechanisms by which ammonium hydroxide affects zeolite nucleation, crystal growth, and framework stability. This includes investigating its impact on the formation of secondary building units (SBUs), the assembly of these units into larger structures, and the final topology of the zeolite framework.
The goal is to develop a comprehensive model that can predict and control zeolite structure based on the concentration and application method of ammonium hydroxide during synthesis. This model would enable the design of zeolites with unprecedented precision, opening new avenues for their application in emerging fields such as nanotechnology and renewable energy.
Furthermore, this research aims to explore the potential of ammonium hydroxide in the synthesis of novel zeolite structures that have not been achievable through conventional methods. By manipulating the structural determinants influenced by ammonium hydroxide, it may be possible to create zeolites with unique properties and functionalities.
Ultimately, this technical investigation seeks to bridge the gap between fundamental understanding and practical application, providing insights that can be translated into improved industrial processes for zeolite production. The findings are expected to contribute significantly to the ongoing efforts to expand the zeolite family and enhance their performance in existing and future applications.
Market Analysis for Zeolite Applications
The zeolite market has experienced significant growth in recent years, driven by the increasing demand for these versatile materials across various industries. Zeolites, with their unique porous structure and ion-exchange properties, have found applications in diverse sectors, including petrochemicals, water treatment, agriculture, and construction.
In the petrochemical industry, zeolites play a crucial role as catalysts in fluid catalytic cracking (FCC) processes, which are essential for the production of gasoline and other valuable products. The growing demand for transportation fuels, particularly in developing economies, has been a key driver for zeolite market expansion in this sector.
Water treatment applications represent another major market for zeolites. Their ability to remove heavy metals, ammonia, and other contaminants from water has led to increased adoption in both industrial and municipal water treatment facilities. As global concerns about water scarcity and quality continue to rise, the demand for zeolites in this sector is expected to grow steadily.
The agricultural sector has also embraced zeolites for their soil amendment properties. These materials can improve soil structure, water retention, and nutrient availability, leading to enhanced crop yields. With the increasing focus on sustainable agriculture and food security, the use of zeolites in this sector is projected to expand further.
In the construction industry, zeolites are utilized as additives in cement and concrete, improving strength and durability while reducing environmental impact. The growing emphasis on green building practices and sustainable construction materials has boosted the demand for zeolites in this sector.
The global zeolite market is segmented by type, with natural and synthetic zeolites each serving specific applications. Natural zeolites, being more cost-effective, dominate in certain sectors like agriculture and water treatment. Synthetic zeolites, offering more precise and tailored properties, are preferred in high-value applications such as catalysts and adsorbents.
Geographically, Asia-Pacific has emerged as the largest market for zeolites, driven by rapid industrialization, urbanization, and environmental concerns in countries like China and India. North America and Europe follow, with mature markets focused on advanced applications and environmental solutions.
The market landscape is characterized by a mix of large multinational corporations and smaller, specialized producers. Key players are investing in research and development to enhance zeolite properties and explore new applications, particularly in emerging fields like gas separation and energy storage.
In the petrochemical industry, zeolites play a crucial role as catalysts in fluid catalytic cracking (FCC) processes, which are essential for the production of gasoline and other valuable products. The growing demand for transportation fuels, particularly in developing economies, has been a key driver for zeolite market expansion in this sector.
Water treatment applications represent another major market for zeolites. Their ability to remove heavy metals, ammonia, and other contaminants from water has led to increased adoption in both industrial and municipal water treatment facilities. As global concerns about water scarcity and quality continue to rise, the demand for zeolites in this sector is expected to grow steadily.
The agricultural sector has also embraced zeolites for their soil amendment properties. These materials can improve soil structure, water retention, and nutrient availability, leading to enhanced crop yields. With the increasing focus on sustainable agriculture and food security, the use of zeolites in this sector is projected to expand further.
In the construction industry, zeolites are utilized as additives in cement and concrete, improving strength and durability while reducing environmental impact. The growing emphasis on green building practices and sustainable construction materials has boosted the demand for zeolites in this sector.
The global zeolite market is segmented by type, with natural and synthetic zeolites each serving specific applications. Natural zeolites, being more cost-effective, dominate in certain sectors like agriculture and water treatment. Synthetic zeolites, offering more precise and tailored properties, are preferred in high-value applications such as catalysts and adsorbents.
Geographically, Asia-Pacific has emerged as the largest market for zeolites, driven by rapid industrialization, urbanization, and environmental concerns in countries like China and India. North America and Europe follow, with mature markets focused on advanced applications and environmental solutions.
The market landscape is characterized by a mix of large multinational corporations and smaller, specialized producers. Key players are investing in research and development to enhance zeolite properties and explore new applications, particularly in emerging fields like gas separation and energy storage.
Current Challenges in Ammonium Hydroxide-based Synthesis
The synthesis of zeolites using ammonium hydroxide faces several significant challenges that researchers and industry professionals are actively working to overcome. One of the primary issues is the precise control of pH during the synthesis process. Ammonium hydroxide, being a weak base, can lead to fluctuations in pH levels, which directly impacts the crystallization kinetics and the final zeolite structure. This variability can result in inconsistent product quality and reduced reproducibility of synthesis outcomes.
Another challenge lies in the volatility of ammonium hydroxide. Its tendency to evaporate during the synthesis process can alter the reaction conditions, leading to changes in the Si/Al ratio and affecting the zeolite framework formation. This volatility also poses environmental and safety concerns, requiring careful handling and specialized equipment for large-scale production.
The interaction between ammonium hydroxide and other synthesis components, such as silica and alumina sources, presents additional complexities. The presence of ammonium ions can influence the solubility and speciation of silicate and aluminate species, affecting the nucleation and growth processes of zeolite crystals. Understanding and controlling these interactions is crucial for tailoring zeolite properties but remains a significant challenge.
Furthermore, the use of ammonium hydroxide in zeolite synthesis often results in the incorporation of ammonium ions into the zeolite structure. While this can be beneficial for certain applications, it may require additional post-synthesis treatments for applications where pure sodium or proton forms are desired. The removal of ammonium ions without compromising the zeolite structure integrity is a delicate process that requires optimization.
The scalability of ammonium hydroxide-based zeolite synthesis is another area of concern. While effective at laboratory scales, translating these processes to industrial production levels presents challenges in maintaining uniform mixing, temperature control, and reaction kinetics. The economic viability of large-scale production using ammonium hydroxide as a structure-directing agent is also under scrutiny, especially when compared to alternative synthesis routes.
Lastly, the environmental impact of ammonium hydroxide use in zeolite synthesis is gaining attention. The potential release of ammonia during the process and the disposal of ammonium-containing waste streams pose environmental challenges. Developing more sustainable and eco-friendly synthesis methods that maintain or improve upon the structural determinants achieved with ammonium hydroxide remains an active area of research in the field of zeolite synthesis.
Another challenge lies in the volatility of ammonium hydroxide. Its tendency to evaporate during the synthesis process can alter the reaction conditions, leading to changes in the Si/Al ratio and affecting the zeolite framework formation. This volatility also poses environmental and safety concerns, requiring careful handling and specialized equipment for large-scale production.
The interaction between ammonium hydroxide and other synthesis components, such as silica and alumina sources, presents additional complexities. The presence of ammonium ions can influence the solubility and speciation of silicate and aluminate species, affecting the nucleation and growth processes of zeolite crystals. Understanding and controlling these interactions is crucial for tailoring zeolite properties but remains a significant challenge.
Furthermore, the use of ammonium hydroxide in zeolite synthesis often results in the incorporation of ammonium ions into the zeolite structure. While this can be beneficial for certain applications, it may require additional post-synthesis treatments for applications where pure sodium or proton forms are desired. The removal of ammonium ions without compromising the zeolite structure integrity is a delicate process that requires optimization.
The scalability of ammonium hydroxide-based zeolite synthesis is another area of concern. While effective at laboratory scales, translating these processes to industrial production levels presents challenges in maintaining uniform mixing, temperature control, and reaction kinetics. The economic viability of large-scale production using ammonium hydroxide as a structure-directing agent is also under scrutiny, especially when compared to alternative synthesis routes.
Lastly, the environmental impact of ammonium hydroxide use in zeolite synthesis is gaining attention. The potential release of ammonia during the process and the disposal of ammonium-containing waste streams pose environmental challenges. Developing more sustainable and eco-friendly synthesis methods that maintain or improve upon the structural determinants achieved with ammonium hydroxide remains an active area of research in the field of zeolite synthesis.
Existing Ammonium Hydroxide Synthesis Methods
01 Chemical structure and properties of ammonium hydroxide
Ammonium hydroxide is a compound formed by the dissolution of ammonia in water. It has the chemical formula NH4OH and is a weak base. The structure consists of ammonium ions (NH4+) and hydroxide ions (OH-) in an aqueous solution. It is commonly used in various industrial and household applications due to its alkaline nature and ability to neutralize acids.- Chemical structure and properties of ammonium hydroxide: Ammonium hydroxide is a compound formed by the dissolution of ammonia in water. It has a molecular formula of NH4OH and is a weak base. The structure consists of an ammonium ion (NH4+) and a hydroxide ion (OH-) in aqueous solution. It is commonly used in various industrial and household applications due to its alkaline nature and ability to neutralize acids.
- Production methods for ammonium hydroxide: Various methods are employed to produce ammonium hydroxide. One common method involves the reaction of ammonia gas with water. Another approach is the electrolysis of ammonium salts. Some processes utilize the reaction of ammonium salts with strong bases to generate ammonium hydroxide. These production methods are optimized for industrial-scale manufacturing to meet the demand for this versatile compound.
- Applications of ammonium hydroxide in chemical processes: Ammonium hydroxide finds extensive use in various chemical processes. It serves as a pH regulator in many industrial applications, including wastewater treatment and textile processing. In the pharmaceutical industry, it is used in the synthesis of certain drugs. Ammonium hydroxide is also employed in the production of household cleaning products and as a reagent in analytical chemistry for qualitative and quantitative analysis.
- Environmental and safety considerations of ammonium hydroxide: The use and handling of ammonium hydroxide require careful consideration of environmental and safety factors. It can be corrosive and irritating to the skin, eyes, and respiratory system. Proper storage, handling, and disposal procedures are essential to minimize environmental impact and ensure worker safety. Regulations often govern its use and transport due to its potential hazards.
- Analytical methods for determining ammonium hydroxide concentration: Various analytical techniques are employed to determine the concentration of ammonium hydroxide in solutions. These include titration methods, spectrophotometric analysis, and ion-selective electrode measurements. Some advanced techniques involve chromatography and mass spectrometry for more precise quantification. The choice of method depends on the required accuracy, sample matrix, and concentration range of interest.
02 Production methods for ammonium hydroxide
Various methods are employed to produce ammonium hydroxide. These include the reaction of ammonia gas with water, electrolysis of ammonium salts, and the decomposition of certain nitrogen-containing compounds. The production process often involves careful control of temperature, pressure, and concentration to achieve the desired purity and strength of the final product.Expand Specific Solutions03 Applications of ammonium hydroxide in industry
Ammonium hydroxide finds widespread use in various industrial applications. It is utilized in the production of textiles, rubber, and leather goods. In the chemical industry, it serves as a pH regulator and a reactant in the synthesis of other compounds. Additionally, it is employed in the manufacture of cleaning products, fertilizers, and in wastewater treatment processes.Expand Specific Solutions04 Environmental and safety considerations
The use and handling of ammonium hydroxide require careful consideration of environmental and safety factors. It can be corrosive and irritating to the skin, eyes, and respiratory system. Proper storage, handling, and disposal procedures are essential to minimize risks. Environmental regulations often govern its use and release to prevent potential harm to ecosystems and water sources.Expand Specific Solutions05 Analytical methods for ammonium hydroxide
Various analytical techniques are employed to determine the concentration and purity of ammonium hydroxide solutions. These methods include titration, spectrophotometry, and ion chromatography. Advanced instrumental techniques such as mass spectrometry and nuclear magnetic resonance spectroscopy may also be used for more detailed structural analysis and characterization of ammonium hydroxide and its derivatives.Expand Specific Solutions
Key Players in Zeolite Industry
The synthesis of zeolites using ammonium hydroxide is a mature field with significant market potential, driven by the growing demand for catalysts and adsorbents in various industries. The competitive landscape is characterized by established players like UOP LLC, BASF Corp., and ExxonMobil Technology & Engineering Co., who have extensive research and development capabilities. These companies are at the forefront of innovation, continuously improving zeolite synthesis techniques and exploring new applications. The market is also seeing increased participation from academic institutions and research organizations, such as the Spanish National Research Council and Jilin University, contributing to the advancement of zeolite technology. As the industry progresses, collaborations between industry and academia are likely to play a crucial role in driving further innovations and expanding the application scope of zeolites.
UOP LLC
Technical Solution: UOP LLC has developed a novel approach for zeolite synthesis using ammonium hydroxide as a structure-directing agent. Their method involves precise control of the NH4OH concentration and pH to manipulate the Si/Al ratio and crystal size of the zeolite framework. By adjusting these parameters, UOP has successfully synthesized high-silica zeolites with improved thermal stability and acid resistance[1]. The company has also implemented a two-step crystallization process, where initial nucleation occurs at lower temperatures, followed by crystal growth at elevated temperatures, resulting in more uniform particle size distribution[3].
Strengths: Precise control over zeolite properties, improved thermal stability, and acid resistance. Weaknesses: Potentially higher production costs due to the two-step process and the need for precise control of synthesis conditions.
BASF Corp.
Technical Solution: BASF Corp. has pioneered a sustainable approach to zeolite synthesis using ammonium hydroxide. Their method incorporates recycled industrial waste streams as silicon and aluminum sources, reducing the environmental impact of zeolite production[2]. BASF's process utilizes a carefully controlled hydrothermal synthesis with NH4OH as both a pH regulator and structure-directing agent. This approach allows for the creation of zeolites with tailored pore sizes and acidic properties, suitable for various catalytic applications[4]. Additionally, BASF has developed a post-synthesis treatment using ammonium hydroxide to enhance the stability and performance of their zeolite catalysts[5].
Strengths: Sustainable production method, tailored zeolite properties for specific applications, and enhanced catalyst stability. Weaknesses: Potential variability in product quality due to the use of waste streams as raw materials.
Structural Determinants in Zeolite Formation
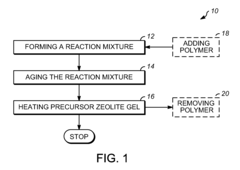
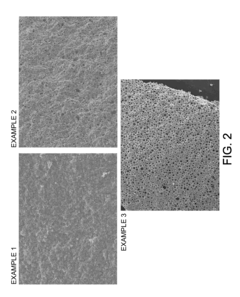
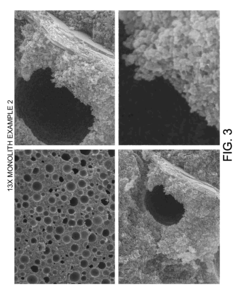
Monolithic zeolite structures with and without hierarchical pore structures and methods for producing the same
PatentInactiveUS20130052126A1
Innovation
- A method for producing monolithic zeolites with hierarchical pore structures using a reaction mixture of silica, alumina, and a cation base, without the need for external solid templates, by aging and heating the mixture to form agglomerated nanocrystalline zeolite crystals with micropores, mesopores, and macropores, allowing for self-assembly and reduced processing complexity.
Zeolite sol and method for preparing the same, composition for forming porous film, porous film and method for forming the same, interlevel insulator film, and semiconductor device
PatentInactiveUS20040091419A1
Innovation
- A zeolite sol with micro-particles of 3 to 15 nm diameter is used, prepared by hydrolyzing a silane compound in the presence of a structure-directing agent and basic catalyst, and added to a silicon-containing composition to form a porous film with enhanced mechanical strength and dielectric properties, allowing for controlled thickness and uniformity.
Environmental Impact of Zeolite Production
The production of zeolites, while beneficial for various industrial applications, has significant environmental implications that warrant careful consideration. The synthesis process, particularly when utilizing ammonium hydroxide, can lead to several environmental concerns. Firstly, the release of ammonia during production contributes to air pollution, potentially causing respiratory issues in nearby communities and impacting local ecosystems. This emission can also contribute to the formation of particulate matter, exacerbating air quality problems in industrial areas.
Water pollution is another critical environmental issue associated with zeolite production. The synthesis process often generates wastewater containing high levels of alkaline substances, dissolved silica, and other chemical residues. If not properly treated, this effluent can alter the pH balance of local water bodies, harming aquatic life and potentially contaminating groundwater resources. The presence of silica in wastewater can also lead to scaling problems in water treatment facilities and natural water systems.
Energy consumption in zeolite production is substantial, contributing to greenhouse gas emissions and climate change. The high-temperature calcination process, often required in zeolite synthesis, demands significant energy input, typically derived from fossil fuels. This not only depletes non-renewable energy resources but also increases the carbon footprint of zeolite manufacturing.
The extraction of raw materials for zeolite synthesis, such as silica and alumina, can lead to habitat destruction and landscape alteration. Mining activities for these materials may result in soil erosion, loss of biodiversity, and disruption of local ecosystems. Furthermore, the transportation of raw materials and finished products adds to the overall environmental impact through increased carbon emissions and potential for accidental spills during transit.
Waste management in zeolite production presents additional environmental challenges. The synthesis process can generate solid waste, including unreacted materials and by-products, which require proper disposal. Improper handling of these wastes can lead to soil contamination and potential leaching of harmful substances into the environment.
To mitigate these environmental impacts, the zeolite industry is increasingly focusing on sustainable production methods. This includes developing more efficient synthesis processes that reduce energy consumption and waste generation. Recycling of process water, implementation of advanced air pollution control technologies, and exploration of alternative, more environmentally friendly raw materials are among the strategies being pursued to enhance the sustainability of zeolite production.
Water pollution is another critical environmental issue associated with zeolite production. The synthesis process often generates wastewater containing high levels of alkaline substances, dissolved silica, and other chemical residues. If not properly treated, this effluent can alter the pH balance of local water bodies, harming aquatic life and potentially contaminating groundwater resources. The presence of silica in wastewater can also lead to scaling problems in water treatment facilities and natural water systems.
Energy consumption in zeolite production is substantial, contributing to greenhouse gas emissions and climate change. The high-temperature calcination process, often required in zeolite synthesis, demands significant energy input, typically derived from fossil fuels. This not only depletes non-renewable energy resources but also increases the carbon footprint of zeolite manufacturing.
The extraction of raw materials for zeolite synthesis, such as silica and alumina, can lead to habitat destruction and landscape alteration. Mining activities for these materials may result in soil erosion, loss of biodiversity, and disruption of local ecosystems. Furthermore, the transportation of raw materials and finished products adds to the overall environmental impact through increased carbon emissions and potential for accidental spills during transit.
Waste management in zeolite production presents additional environmental challenges. The synthesis process can generate solid waste, including unreacted materials and by-products, which require proper disposal. Improper handling of these wastes can lead to soil contamination and potential leaching of harmful substances into the environment.
To mitigate these environmental impacts, the zeolite industry is increasingly focusing on sustainable production methods. This includes developing more efficient synthesis processes that reduce energy consumption and waste generation. Recycling of process water, implementation of advanced air pollution control technologies, and exploration of alternative, more environmentally friendly raw materials are among the strategies being pursued to enhance the sustainability of zeolite production.
Zeolite Characterization Techniques
Zeolite characterization techniques play a crucial role in understanding the structural determinants influenced by ammonium hydroxide in zeolite synthesis. These techniques provide valuable insights into the physical and chemical properties of zeolites, enabling researchers to optimize synthesis conditions and tailor zeolite structures for specific applications.
X-ray diffraction (XRD) is a fundamental technique for zeolite characterization, offering information on crystal structure, phase purity, and crystallinity. By analyzing the diffraction patterns, researchers can identify the zeolite framework type and assess the impact of ammonium hydroxide on the formation of specific structural features.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) provide detailed information on zeolite morphology and particle size distribution. These techniques are particularly useful for examining the influence of ammonium hydroxide on crystal growth and the development of hierarchical structures in zeolites.
Nitrogen adsorption-desorption isotherms are employed to determine the surface area, pore volume, and pore size distribution of zeolites. This technique helps researchers understand how ammonium hydroxide affects the porosity and surface properties of the synthesized materials, which are critical factors in many applications.
Fourier-transform infrared spectroscopy (FTIR) is used to investigate the chemical composition and bonding environment within zeolites. By analyzing the vibrational modes of framework atoms and guest species, FTIR can reveal the impact of ammonium hydroxide on the formation of specific structural units and the incorporation of template molecules.
Magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy provides detailed information on the local chemical environment of atoms within the zeolite framework. This technique is particularly useful for studying the distribution of aluminum and silicon atoms in the structure, which can be influenced by the presence of ammonium hydroxide during synthesis.
Temperature-programmed desorption (TPD) and thermogravimetric analysis (TGA) are valuable techniques for investigating the thermal stability and acidity of zeolites. These methods can reveal how ammonium hydroxide affects the distribution and strength of acid sites, as well as the thermal behavior of the synthesized materials.
Advanced synchrotron-based techniques, such as X-ray absorption spectroscopy (XAS) and small-angle X-ray scattering (SAXS), offer additional insights into the local structure and long-range order of zeolites. These techniques can provide valuable information on the role of ammonium hydroxide in directing the formation of specific structural features and hierarchical architectures.
By combining these characterization techniques, researchers can gain a comprehensive understanding of how ammonium hydroxide influences the structural determinants of zeolites during synthesis. This knowledge is essential for developing tailored zeolite materials with optimized properties for various applications in catalysis, separation, and adsorption processes.
X-ray diffraction (XRD) is a fundamental technique for zeolite characterization, offering information on crystal structure, phase purity, and crystallinity. By analyzing the diffraction patterns, researchers can identify the zeolite framework type and assess the impact of ammonium hydroxide on the formation of specific structural features.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) provide detailed information on zeolite morphology and particle size distribution. These techniques are particularly useful for examining the influence of ammonium hydroxide on crystal growth and the development of hierarchical structures in zeolites.
Nitrogen adsorption-desorption isotherms are employed to determine the surface area, pore volume, and pore size distribution of zeolites. This technique helps researchers understand how ammonium hydroxide affects the porosity and surface properties of the synthesized materials, which are critical factors in many applications.
Fourier-transform infrared spectroscopy (FTIR) is used to investigate the chemical composition and bonding environment within zeolites. By analyzing the vibrational modes of framework atoms and guest species, FTIR can reveal the impact of ammonium hydroxide on the formation of specific structural units and the incorporation of template molecules.
Magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy provides detailed information on the local chemical environment of atoms within the zeolite framework. This technique is particularly useful for studying the distribution of aluminum and silicon atoms in the structure, which can be influenced by the presence of ammonium hydroxide during synthesis.
Temperature-programmed desorption (TPD) and thermogravimetric analysis (TGA) are valuable techniques for investigating the thermal stability and acidity of zeolites. These methods can reveal how ammonium hydroxide affects the distribution and strength of acid sites, as well as the thermal behavior of the synthesized materials.
Advanced synchrotron-based techniques, such as X-ray absorption spectroscopy (XAS) and small-angle X-ray scattering (SAXS), offer additional insights into the local structure and long-range order of zeolites. These techniques can provide valuable information on the role of ammonium hydroxide in directing the formation of specific structural features and hierarchical architectures.
By combining these characterization techniques, researchers can gain a comprehensive understanding of how ammonium hydroxide influences the structural determinants of zeolites during synthesis. This knowledge is essential for developing tailored zeolite materials with optimized properties for various applications in catalysis, separation, and adsorption processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!