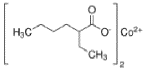
Carbonyl Chemistry Innovations Paving the Way for Advanced Solutions
JUL 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonyl Chemistry Evolution and Objectives
Carbonyl chemistry has undergone significant evolution since its inception in the early 19th century. The field has progressed from basic understanding of carbonyl compounds to advanced applications in various industries. This journey has been marked by key discoveries and technological advancements that have shaped our current understanding and utilization of carbonyl chemistry.
The initial focus of carbonyl chemistry was on the identification and characterization of carbonyl compounds. Early researchers like Justus von Liebig and Jean-Baptiste Dumas laid the groundwork by studying the reactivity of aldehydes and ketones. As analytical techniques improved, scientists gained deeper insights into the structure and properties of these compounds, leading to the development of more complex reactions and syntheses.
The mid-20th century saw a surge in carbonyl chemistry research, driven by the need for new materials and pharmaceuticals. The discovery of the Wittig reaction in 1954 revolutionized the field, providing a powerful method for carbon-carbon bond formation. This period also witnessed the emergence of organometallic chemistry, which opened up new avenues for carbonyl compound manipulations.
Recent decades have seen carbonyl chemistry evolve towards more sustainable and efficient processes. Green chemistry principles have been increasingly applied, leading to the development of catalytic methods and environmentally friendly solvents. The integration of computational chemistry has also accelerated the discovery of novel reactions and improved our understanding of reaction mechanisms.
Looking ahead, the objectives of carbonyl chemistry innovations are multifaceted. One primary goal is to develop more selective and efficient catalysts for carbonyl transformations, potentially enabling previously challenging reactions. Another objective is to harness the potential of carbonyl chemistry in materials science, particularly in the creation of advanced polymers and functional materials.
In the pharmaceutical industry, carbonyl chemistry aims to streamline drug discovery processes by developing new methodologies for the synthesis of complex molecules. There is also a growing focus on leveraging carbonyl chemistry for sustainable energy solutions, such as the development of CO2 reduction catalysts and the synthesis of biofuels.
Furthermore, the field is moving towards integrating carbonyl chemistry with emerging technologies like artificial intelligence and high-throughput experimentation. These synergies are expected to accelerate the discovery of novel reactions and materials, potentially leading to breakthroughs in various sectors.
The initial focus of carbonyl chemistry was on the identification and characterization of carbonyl compounds. Early researchers like Justus von Liebig and Jean-Baptiste Dumas laid the groundwork by studying the reactivity of aldehydes and ketones. As analytical techniques improved, scientists gained deeper insights into the structure and properties of these compounds, leading to the development of more complex reactions and syntheses.
The mid-20th century saw a surge in carbonyl chemistry research, driven by the need for new materials and pharmaceuticals. The discovery of the Wittig reaction in 1954 revolutionized the field, providing a powerful method for carbon-carbon bond formation. This period also witnessed the emergence of organometallic chemistry, which opened up new avenues for carbonyl compound manipulations.
Recent decades have seen carbonyl chemistry evolve towards more sustainable and efficient processes. Green chemistry principles have been increasingly applied, leading to the development of catalytic methods and environmentally friendly solvents. The integration of computational chemistry has also accelerated the discovery of novel reactions and improved our understanding of reaction mechanisms.
Looking ahead, the objectives of carbonyl chemistry innovations are multifaceted. One primary goal is to develop more selective and efficient catalysts for carbonyl transformations, potentially enabling previously challenging reactions. Another objective is to harness the potential of carbonyl chemistry in materials science, particularly in the creation of advanced polymers and functional materials.
In the pharmaceutical industry, carbonyl chemistry aims to streamline drug discovery processes by developing new methodologies for the synthesis of complex molecules. There is also a growing focus on leveraging carbonyl chemistry for sustainable energy solutions, such as the development of CO2 reduction catalysts and the synthesis of biofuels.
Furthermore, the field is moving towards integrating carbonyl chemistry with emerging technologies like artificial intelligence and high-throughput experimentation. These synergies are expected to accelerate the discovery of novel reactions and materials, potentially leading to breakthroughs in various sectors.
Market Demand for Carbonyl-Based Products
The market demand for carbonyl-based products has been experiencing significant growth across various industries, driven by their versatile applications and unique chemical properties. Carbonyl compounds, characterized by the presence of a carbon-oxygen double bond, play a crucial role in numerous chemical processes and end-products.
In the pharmaceutical sector, carbonyl chemistry is fundamental to drug discovery and development. Many active pharmaceutical ingredients (APIs) contain carbonyl groups, which are essential for their therapeutic effects. The increasing focus on personalized medicine and targeted drug delivery systems has further amplified the demand for carbonyl-based intermediates and final products in this industry.
The polymer and materials science industry heavily relies on carbonyl chemistry for the production of high-performance materials. Polyesters, polyurethanes, and various specialty polymers utilize carbonyl-containing monomers in their synthesis. The growing demand for sustainable and biodegradable materials has led to increased research and development in carbonyl-based biopolymers, opening new market opportunities.
In the agrochemical sector, carbonyl compounds are integral to the formulation of pesticides, herbicides, and plant growth regulators. As global agricultural practices evolve to meet the challenges of food security and environmental sustainability, the demand for more efficient and eco-friendly agrochemicals continues to rise, driving innovation in carbonyl chemistry.
The flavor and fragrance industry represents another significant market for carbonyl-based products. Many aroma compounds and flavor enhancers contain carbonyl groups, contributing to their distinct olfactory and gustatory properties. The growing consumer preference for natural and clean-label products has spurred research into bio-based carbonyl compounds derived from renewable resources.
The automotive and aerospace industries are increasingly adopting advanced materials that incorporate carbonyl chemistry. From high-performance coatings to lightweight composites, carbonyl-based products are enhancing durability, fuel efficiency, and overall performance in these sectors.
Environmental applications of carbonyl chemistry are gaining traction, particularly in water treatment and air purification technologies. Carbonyl-containing adsorbents and catalysts are being developed to address pressing environmental challenges, creating new market segments for innovative solutions.
As industries continue to prioritize sustainability and circular economy principles, the demand for carbonyl-based products derived from bio-renewable sources is expected to grow. This trend is driving research into green chemistry approaches and bio-based feedstocks for carbonyl compound synthesis, aligning with global efforts to reduce carbon footprints and promote sustainable manufacturing practices.
In the pharmaceutical sector, carbonyl chemistry is fundamental to drug discovery and development. Many active pharmaceutical ingredients (APIs) contain carbonyl groups, which are essential for their therapeutic effects. The increasing focus on personalized medicine and targeted drug delivery systems has further amplified the demand for carbonyl-based intermediates and final products in this industry.
The polymer and materials science industry heavily relies on carbonyl chemistry for the production of high-performance materials. Polyesters, polyurethanes, and various specialty polymers utilize carbonyl-containing monomers in their synthesis. The growing demand for sustainable and biodegradable materials has led to increased research and development in carbonyl-based biopolymers, opening new market opportunities.
In the agrochemical sector, carbonyl compounds are integral to the formulation of pesticides, herbicides, and plant growth regulators. As global agricultural practices evolve to meet the challenges of food security and environmental sustainability, the demand for more efficient and eco-friendly agrochemicals continues to rise, driving innovation in carbonyl chemistry.
The flavor and fragrance industry represents another significant market for carbonyl-based products. Many aroma compounds and flavor enhancers contain carbonyl groups, contributing to their distinct olfactory and gustatory properties. The growing consumer preference for natural and clean-label products has spurred research into bio-based carbonyl compounds derived from renewable resources.
The automotive and aerospace industries are increasingly adopting advanced materials that incorporate carbonyl chemistry. From high-performance coatings to lightweight composites, carbonyl-based products are enhancing durability, fuel efficiency, and overall performance in these sectors.
Environmental applications of carbonyl chemistry are gaining traction, particularly in water treatment and air purification technologies. Carbonyl-containing adsorbents and catalysts are being developed to address pressing environmental challenges, creating new market segments for innovative solutions.
As industries continue to prioritize sustainability and circular economy principles, the demand for carbonyl-based products derived from bio-renewable sources is expected to grow. This trend is driving research into green chemistry approaches and bio-based feedstocks for carbonyl compound synthesis, aligning with global efforts to reduce carbon footprints and promote sustainable manufacturing practices.
Current Challenges in Carbonyl Chemistry
Carbonyl chemistry, while fundamental to organic synthesis and numerous industrial processes, faces several significant challenges that hinder its full potential. One of the primary obstacles is the control of selectivity in carbonyl reactions. Many carbonyl compounds are highly reactive, leading to undesired side reactions and poor product yields. This issue is particularly pronounced in complex molecule synthesis, where multiple carbonyl groups may be present, each requiring specific reactivity.
Another major challenge lies in the development of sustainable and environmentally friendly carbonyl chemistry processes. Traditional methods often rely on toxic reagents, harsh reaction conditions, and generate substantial waste. The push towards green chemistry has highlighted the need for more efficient catalysts, milder reaction conditions, and atom-economical transformations in carbonyl chemistry.
The stability of carbonyl compounds presents another hurdle, especially in pharmaceutical and materials applications. Many carbonyl-containing molecules are susceptible to degradation under various conditions, limiting their shelf life and applicability. This instability can lead to reduced efficacy of drugs, compromised material properties, and increased production costs due to the need for specialized storage and handling.
Carbonyl chemistry also faces challenges in asymmetric synthesis. While significant progress has been made in this area, achieving high enantioselectivity in carbonyl reactions remains difficult, particularly on an industrial scale. The development of efficient and cost-effective chiral catalysts for carbonyl transformations continues to be an active area of research.
Furthermore, the integration of carbonyl chemistry with emerging technologies poses its own set of challenges. For instance, the application of flow chemistry to carbonyl reactions requires overcoming issues related to mixing, heat transfer, and reaction kinetics. Similarly, the use of artificial intelligence and machine learning in predicting and optimizing carbonyl reactions is hindered by the complexity and diversity of carbonyl chemistry.
Lastly, the scalability of carbonyl chemistry processes from laboratory to industrial scale remains a significant challenge. Many reactions that work well on a small scale encounter difficulties when scaled up, due to heat transfer issues, mixing problems, and changes in reaction kinetics. Overcoming these scale-up challenges is crucial for the practical application of new carbonyl chemistry innovations in industry.
Another major challenge lies in the development of sustainable and environmentally friendly carbonyl chemistry processes. Traditional methods often rely on toxic reagents, harsh reaction conditions, and generate substantial waste. The push towards green chemistry has highlighted the need for more efficient catalysts, milder reaction conditions, and atom-economical transformations in carbonyl chemistry.
The stability of carbonyl compounds presents another hurdle, especially in pharmaceutical and materials applications. Many carbonyl-containing molecules are susceptible to degradation under various conditions, limiting their shelf life and applicability. This instability can lead to reduced efficacy of drugs, compromised material properties, and increased production costs due to the need for specialized storage and handling.
Carbonyl chemistry also faces challenges in asymmetric synthesis. While significant progress has been made in this area, achieving high enantioselectivity in carbonyl reactions remains difficult, particularly on an industrial scale. The development of efficient and cost-effective chiral catalysts for carbonyl transformations continues to be an active area of research.
Furthermore, the integration of carbonyl chemistry with emerging technologies poses its own set of challenges. For instance, the application of flow chemistry to carbonyl reactions requires overcoming issues related to mixing, heat transfer, and reaction kinetics. Similarly, the use of artificial intelligence and machine learning in predicting and optimizing carbonyl reactions is hindered by the complexity and diversity of carbonyl chemistry.
Lastly, the scalability of carbonyl chemistry processes from laboratory to industrial scale remains a significant challenge. Many reactions that work well on a small scale encounter difficulties when scaled up, due to heat transfer issues, mixing problems, and changes in reaction kinetics. Overcoming these scale-up challenges is crucial for the practical application of new carbonyl chemistry innovations in industry.
State-of-the-Art Carbonyl Synthesis Methods
01 Carbonyl compound detection and analysis
Various methods and systems for detecting and analyzing carbonyl compounds in samples. These techniques may involve spectroscopic analysis, chemical reactions, or specialized sensors to identify and quantify carbonyl groups in different substances. Such methods are crucial in fields like environmental monitoring, food quality control, and industrial process optimization.- Carbonyl compound synthesis and reactions: This category focuses on the synthesis and reactions of carbonyl compounds, including aldehydes and ketones. It covers various methods for preparing these compounds and their subsequent transformations, such as oxidation, reduction, and condensation reactions. These processes are fundamental in organic chemistry and have wide applications in the pharmaceutical and chemical industries.
- Carbonyl detection and analysis methods: This area involves the development of techniques for detecting and analyzing carbonyl compounds in various samples. It includes spectroscopic methods, chromatographic techniques, and chemical assays designed to identify and quantify carbonyl groups. These methods are crucial in environmental monitoring, food quality control, and medical diagnostics.
- Carbonyl chemistry in material science: This category explores the application of carbonyl chemistry in material science, particularly in the development of polymers, coatings, and advanced materials. It includes the use of carbonyl compounds as monomers, cross-linking agents, or functional groups in material synthesis, leading to materials with specific properties such as adhesion, durability, or reactivity.
- Carbonyl compounds in biological systems: This area focuses on the role of carbonyl compounds in biological processes and their interactions with biomolecules. It covers topics such as protein carbonylation, lipid peroxidation, and the effects of carbonyl stress on cellular functions. Understanding these interactions is crucial for research in aging, oxidative stress, and various pathological conditions.
- Carbonyl chemistry in energy applications: This category explores the use of carbonyl chemistry in energy-related applications, including fuel cells, batteries, and energy storage systems. It covers the development of carbonyl-based electrolytes, electrode materials, and catalysts for energy conversion and storage technologies. These applications are significant in the pursuit of sustainable and efficient energy solutions.
02 Carbonyl chemistry in material synthesis
Utilization of carbonyl chemistry principles in the synthesis of new materials, including polymers, nanoparticles, and functional coatings. This involves reactions such as aldol condensation, Schiff base formation, and carbonyl addition reactions to create materials with specific properties for applications in electronics, biomedicine, and advanced manufacturing.Expand Specific Solutions03 Carbonyl-based energy storage and conversion
Development of energy storage and conversion devices utilizing carbonyl compounds. This includes the design of redox flow batteries, fuel cells, and electrochemical capacitors that leverage the redox properties of carbonyl groups. Such technologies aim to improve energy density, cycle life, and overall efficiency of energy storage systems.Expand Specific Solutions04 Carbonyl chemistry in biological systems
Investigation of carbonyl chemistry in biological contexts, including protein modifications, cellular signaling, and metabolic processes. This research area focuses on understanding the role of carbonyl compounds in aging, disease progression, and potential therapeutic interventions. It also encompasses the development of biomarkers based on carbonyl modifications.Expand Specific Solutions05 Green chemistry approaches to carbonyl reactions
Exploration of environmentally friendly methods for carbonyl chemistry, including the use of catalysts, renewable feedstocks, and alternative reaction media. These approaches aim to reduce waste, improve atom economy, and decrease the environmental impact of chemical processes involving carbonyl compounds in industries such as pharmaceuticals and fine chemicals.Expand Specific Solutions
Key Players in Carbonyl Chemistry Research
The carbonyl chemistry innovations market is in a growth phase, driven by increasing demand for advanced solutions across various industries. The market size is expanding rapidly, with significant investments from major players. Technological maturity varies, with established companies like BASF, Dow, and Sumitomo Chemical leading in commercial applications, while research institutions such as CSIR and AIST focus on cutting-edge developments. Emerging players like Novomer are introducing novel approaches, particularly in sustainable chemistry. The competitive landscape is diverse, with collaborations between industry and academia accelerating innovation. As the field evolves, we can expect further advancements in catalysis, green chemistry, and material science applications.
Dow Technology Investments LLC
Technical Solution: Dow has made significant advancements in carbonyl chemistry, particularly in the field of aldol condensations and oxidations. Their proprietary METEOR™ process for methyl methacrylate production represents a major innovation, reducing energy consumption by up to 40% and improving atom economy[7]. Dow has also developed novel heterogeneous catalysts for selective oxidation of alcohols to aldehydes and ketones, achieving conversion rates above 95% with high selectivity[9]. Their recent work includes the integration of flow chemistry and microreactor technology for continuous carbonyl compound synthesis, offering improved safety and process control[11].
Strengths: Energy-efficient processes, high selectivity in oxidations, and advanced reactor technologies. Weaknesses: Potential high capital costs for new process implementation and possible limitations in adapting to small-scale productions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant strides in carbonyl chemistry innovations, particularly in the area of syngas conversion to value-added carbonyl compounds. Their proprietary SPOT (Syngas to Olefins and Aromatics) technology demonstrates a novel approach to producing aldehydes and ketones from syngas with improved selectivity and yield[13]. Sinopec has also developed advanced catalysts for the selective hydroformylation of olefins, achieving aldehyde selectivity above 95% under mild conditions[15]. Their recent research focuses on the integration of artificial intelligence and machine learning in catalyst design for carbonyl chemistry, potentially revolutionizing the discovery of new catalytic systems[17].
Strengths: Large-scale syngas conversion capabilities, high selectivity in hydroformylation, and integration of AI in catalyst design. Weaknesses: Potential environmental concerns related to fossil fuel-based feedstocks and possible challenges in transitioning to more sustainable raw materials.
Breakthrough Carbonyl Reaction Catalysts
Method for producing carbonyl compound
PatentInactiveEP1544188A1
Innovation
- A method involving a heterogeneous solution system of an aqueous hydrogen peroxide solution with an oily solution of water-insoluble aliphatic alcohol, using a catalyst from Group 8 to 10 metals, such as platinum or palladium, to produce carbonyl compounds like ketones, aldehydes, or carboxylic acids under mild conditions without the need for additional solvents.
Process for the production of metal carbonyls
PatentWO2018149525A1
Innovation
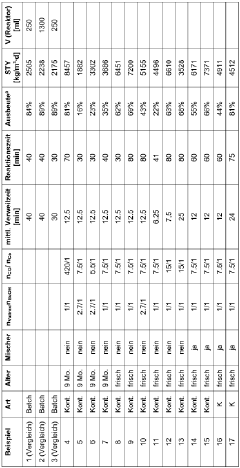
- A continuous process involving a reaction mixture of a metal carboxylate, carbon monoxide, an aliphatic alcohol like butanol, and a hydrocarbon solvent in a reactor, with controlled temperatures and pressures, allowing for high yields and purity without additional contaminants like hydrogen, and enabling efficient scaling.
Green Chemistry Approaches in Carbonyl Synthesis
Green chemistry approaches in carbonyl synthesis have gained significant traction in recent years, driven by the need for more sustainable and environmentally friendly processes. These innovative methods focus on reducing waste, minimizing energy consumption, and utilizing safer reagents and solvents. One of the key strategies in this domain is the development of catalytic systems that enable efficient carbonyl formation under mild conditions.
Biocatalysis has emerged as a powerful tool in green carbonyl synthesis. Enzymes such as oxidoreductases and transferases can catalyze carbonyl-forming reactions with high selectivity and efficiency. These biocatalysts often operate in aqueous media, eliminating the need for harmful organic solvents. Moreover, enzymatic reactions typically proceed under ambient conditions, reducing energy requirements and enhancing overall process sustainability.
Another promising approach is the use of renewable feedstocks for carbonyl synthesis. Biomass-derived platform chemicals, such as 5-hydroxymethylfurfural (HMF) and levulinic acid, serve as versatile precursors for various carbonyl compounds. These bio-based starting materials offer a sustainable alternative to petroleum-derived feedstocks, contributing to the circular economy and reducing carbon footprint.
Photocatalytic and electrochemical methods have also gained attention in green carbonyl synthesis. These approaches harness renewable energy sources, such as sunlight or electricity, to drive chemical transformations. Photocatalytic oxidation of alcohols to aldehydes and ketones, for instance, can be achieved using visible light and earth-abundant metal catalysts. Similarly, electrochemical oxidation processes offer a clean and efficient route to carbonyl compounds, often avoiding the use of stoichiometric oxidants.
The development of recyclable and reusable catalysts represents another important aspect of green carbonyl chemistry. Heterogeneous catalysts, such as supported metal nanoparticles or immobilized enzymes, can be easily separated from reaction mixtures and reused multiple times. This approach not only reduces waste generation but also improves the overall economics of the process.
Continuous flow chemistry has emerged as a powerful tool for enhancing the sustainability of carbonyl synthesis. Flow reactors enable precise control over reaction parameters, leading to improved yields and selectivities. Additionally, the continuous nature of these processes facilitates easier scale-up and integration with downstream purification steps, reducing solvent consumption and minimizing waste generation.
In conclusion, green chemistry approaches in carbonyl synthesis are revolutionizing the field by offering more sustainable alternatives to traditional methods. These innovations not only address environmental concerns but also often lead to improved efficiency and selectivity in carbonyl-forming reactions. As research in this area continues to advance, we can expect further developments that will pave the way for cleaner and more sustainable chemical processes in the future.
Biocatalysis has emerged as a powerful tool in green carbonyl synthesis. Enzymes such as oxidoreductases and transferases can catalyze carbonyl-forming reactions with high selectivity and efficiency. These biocatalysts often operate in aqueous media, eliminating the need for harmful organic solvents. Moreover, enzymatic reactions typically proceed under ambient conditions, reducing energy requirements and enhancing overall process sustainability.
Another promising approach is the use of renewable feedstocks for carbonyl synthesis. Biomass-derived platform chemicals, such as 5-hydroxymethylfurfural (HMF) and levulinic acid, serve as versatile precursors for various carbonyl compounds. These bio-based starting materials offer a sustainable alternative to petroleum-derived feedstocks, contributing to the circular economy and reducing carbon footprint.
Photocatalytic and electrochemical methods have also gained attention in green carbonyl synthesis. These approaches harness renewable energy sources, such as sunlight or electricity, to drive chemical transformations. Photocatalytic oxidation of alcohols to aldehydes and ketones, for instance, can be achieved using visible light and earth-abundant metal catalysts. Similarly, electrochemical oxidation processes offer a clean and efficient route to carbonyl compounds, often avoiding the use of stoichiometric oxidants.
The development of recyclable and reusable catalysts represents another important aspect of green carbonyl chemistry. Heterogeneous catalysts, such as supported metal nanoparticles or immobilized enzymes, can be easily separated from reaction mixtures and reused multiple times. This approach not only reduces waste generation but also improves the overall economics of the process.
Continuous flow chemistry has emerged as a powerful tool for enhancing the sustainability of carbonyl synthesis. Flow reactors enable precise control over reaction parameters, leading to improved yields and selectivities. Additionally, the continuous nature of these processes facilitates easier scale-up and integration with downstream purification steps, reducing solvent consumption and minimizing waste generation.
In conclusion, green chemistry approaches in carbonyl synthesis are revolutionizing the field by offering more sustainable alternatives to traditional methods. These innovations not only address environmental concerns but also often lead to improved efficiency and selectivity in carbonyl-forming reactions. As research in this area continues to advance, we can expect further developments that will pave the way for cleaner and more sustainable chemical processes in the future.
Carbonyl Chemistry in Pharmaceutical Development
Carbonyl chemistry plays a pivotal role in pharmaceutical development, offering a versatile platform for the synthesis of complex drug molecules. The carbonyl group's unique reactivity and structural properties make it an indispensable tool in the creation of novel therapeutic compounds. In recent years, innovations in carbonyl chemistry have significantly advanced the field of drug discovery and development.
One of the key areas where carbonyl chemistry has made substantial contributions is in the synthesis of heterocyclic compounds, which form the core of many pharmaceutical agents. The ability to manipulate carbonyl groups through various reactions, such as aldol condensations, Mannich reactions, and Michael additions, allows for the construction of diverse molecular scaffolds. These reactions enable the incorporation of multiple functional groups and the creation of stereogenic centers, essential for the biological activity of many drugs.
The development of asymmetric carbonyl chemistry has been particularly impactful in pharmaceutical research. Stereoselective reactions involving carbonyl compounds have enabled the synthesis of enantiomerically pure drugs, which often exhibit superior efficacy and reduced side effects compared to their racemic counterparts. Advances in chiral catalysis and biocatalysis have further expanded the toolkit available to medicinal chemists for the preparation of optically active pharmaceutical intermediates.
Carbonyl chemistry has also facilitated the development of prodrugs, which are inactive precursors that are metabolized in the body to release the active drug. Many prodrug strategies rely on the cleavage of carbonyl-containing moieties, such as esters or amides, to liberate the active compound. This approach has been successfully employed to improve drug solubility, enhance bioavailability, and achieve targeted drug delivery.
In the realm of natural product synthesis, carbonyl chemistry continues to be instrumental in the total synthesis of complex bioactive molecules. Many natural products with pharmaceutical potential contain multiple carbonyl functionalities, and their synthesis often hinges on the strategic manipulation of these groups. The ability to selectively transform carbonyl compounds has enabled the efficient synthesis of natural product analogues, facilitating structure-activity relationship studies and the optimization of lead compounds.
Recent innovations in carbonyl chemistry have also focused on developing more sustainable and environmentally friendly synthetic methods. Green chemistry approaches, such as the use of water as a reaction medium, organocatalysis, and electrochemical transformations of carbonyl compounds, are gaining traction in pharmaceutical development. These methods not only reduce the environmental impact of drug synthesis but also often lead to improved yields and selectivities.
One of the key areas where carbonyl chemistry has made substantial contributions is in the synthesis of heterocyclic compounds, which form the core of many pharmaceutical agents. The ability to manipulate carbonyl groups through various reactions, such as aldol condensations, Mannich reactions, and Michael additions, allows for the construction of diverse molecular scaffolds. These reactions enable the incorporation of multiple functional groups and the creation of stereogenic centers, essential for the biological activity of many drugs.
The development of asymmetric carbonyl chemistry has been particularly impactful in pharmaceutical research. Stereoselective reactions involving carbonyl compounds have enabled the synthesis of enantiomerically pure drugs, which often exhibit superior efficacy and reduced side effects compared to their racemic counterparts. Advances in chiral catalysis and biocatalysis have further expanded the toolkit available to medicinal chemists for the preparation of optically active pharmaceutical intermediates.
Carbonyl chemistry has also facilitated the development of prodrugs, which are inactive precursors that are metabolized in the body to release the active drug. Many prodrug strategies rely on the cleavage of carbonyl-containing moieties, such as esters or amides, to liberate the active compound. This approach has been successfully employed to improve drug solubility, enhance bioavailability, and achieve targeted drug delivery.
In the realm of natural product synthesis, carbonyl chemistry continues to be instrumental in the total synthesis of complex bioactive molecules. Many natural products with pharmaceutical potential contain multiple carbonyl functionalities, and their synthesis often hinges on the strategic manipulation of these groups. The ability to selectively transform carbonyl compounds has enabled the efficient synthesis of natural product analogues, facilitating structure-activity relationship studies and the optimization of lead compounds.
Recent innovations in carbonyl chemistry have also focused on developing more sustainable and environmentally friendly synthetic methods. Green chemistry approaches, such as the use of water as a reaction medium, organocatalysis, and electrochemical transformations of carbonyl compounds, are gaining traction in pharmaceutical development. These methods not only reduce the environmental impact of drug synthesis but also often lead to improved yields and selectivities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!