Chemical Reactivity in Alkaline Borosilicate Glass Systems
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkaline Borosilicate Glass Chemistry Evolution
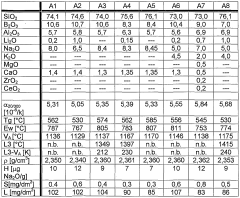
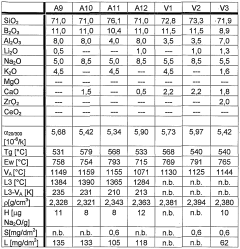
The evolution of alkaline borosilicate glass chemistry has been a journey of continuous refinement and innovation. Initially developed in the early 20th century, these glasses were primarily designed for their exceptional thermal and chemical durability. The fundamental composition typically includes silica (SiO2) as the primary network former, boron oxide (B2O3) as a flux and network former, and alkali oxides (Na2O, K2O) as network modifiers.
In the 1950s and 1960s, significant advancements were made in understanding the role of boron in the glass network. Researchers discovered that boron could exist in both three-fold and four-fold coordination, leading to the development of more complex and tailored glass compositions. This period also saw the introduction of aluminum oxide (Al2O3) as a crucial component, enhancing the chemical durability of the glass.
The 1970s and 1980s marked a pivotal era in alkaline borosilicate glass chemistry, driven by the nuclear waste immobilization needs. Scientists focused on optimizing glass compositions to effectively incorporate radioactive waste elements while maintaining long-term stability. This led to the development of highly resistant glass formulations with increased waste loading capacities.
Towards the end of the 20th century, the focus shifted towards understanding and controlling the leaching behavior of these glasses in various environments. Advanced analytical techniques, such as X-ray absorption spectroscopy and nuclear magnetic resonance, provided deeper insights into the glass structure and the behavior of different elements within the network.
In recent years, the chemistry of alkaline borosilicate glasses has evolved to address specific technological needs. For instance, the development of bioactive glasses for medical applications has led to new compositions that promote controlled dissolution and bone regeneration. Additionally, the increasing demand for high-performance optical materials has driven research into ultra-low expansion glasses and glasses with tailored refractive indices.
The 21st century has seen a surge in computational modeling of glass structures and properties. These advanced simulations have enabled researchers to predict glass behavior and optimize compositions without extensive experimental trials. This approach has significantly accelerated the development of new glass formulations with enhanced properties for specific applications.
Current trends in alkaline borosilicate glass chemistry focus on sustainability and energy efficiency. Researchers are exploring ways to reduce melting temperatures and incorporate recycled materials without compromising glass quality. There is also growing interest in developing glasses with self-healing properties and those capable of responding to external stimuli, opening up new possibilities for smart materials.
In the 1950s and 1960s, significant advancements were made in understanding the role of boron in the glass network. Researchers discovered that boron could exist in both three-fold and four-fold coordination, leading to the development of more complex and tailored glass compositions. This period also saw the introduction of aluminum oxide (Al2O3) as a crucial component, enhancing the chemical durability of the glass.
The 1970s and 1980s marked a pivotal era in alkaline borosilicate glass chemistry, driven by the nuclear waste immobilization needs. Scientists focused on optimizing glass compositions to effectively incorporate radioactive waste elements while maintaining long-term stability. This led to the development of highly resistant glass formulations with increased waste loading capacities.
Towards the end of the 20th century, the focus shifted towards understanding and controlling the leaching behavior of these glasses in various environments. Advanced analytical techniques, such as X-ray absorption spectroscopy and nuclear magnetic resonance, provided deeper insights into the glass structure and the behavior of different elements within the network.
In recent years, the chemistry of alkaline borosilicate glasses has evolved to address specific technological needs. For instance, the development of bioactive glasses for medical applications has led to new compositions that promote controlled dissolution and bone regeneration. Additionally, the increasing demand for high-performance optical materials has driven research into ultra-low expansion glasses and glasses with tailored refractive indices.
The 21st century has seen a surge in computational modeling of glass structures and properties. These advanced simulations have enabled researchers to predict glass behavior and optimize compositions without extensive experimental trials. This approach has significantly accelerated the development of new glass formulations with enhanced properties for specific applications.
Current trends in alkaline borosilicate glass chemistry focus on sustainability and energy efficiency. Researchers are exploring ways to reduce melting temperatures and incorporate recycled materials without compromising glass quality. There is also growing interest in developing glasses with self-healing properties and those capable of responding to external stimuli, opening up new possibilities for smart materials.
Industrial Applications and Market Demand
The alkaline borosilicate glass systems have found extensive applications across various industries, driving significant market demand. In the nuclear waste management sector, these glass systems play a crucial role in the vitrification process, where radioactive waste is immobilized within a glass matrix. This application has seen steady growth due to the increasing need for safe and long-term storage solutions for nuclear waste.
The electronics industry has also embraced alkaline borosilicate glass systems, particularly in the production of display panels and touchscreens. The chemical reactivity of these glasses allows for the creation of high-quality, durable surfaces that can withstand frequent use and environmental factors. As the demand for smart devices continues to rise, the market for specialized glass components is expected to expand correspondingly.
In the pharmaceutical and laboratory equipment sector, alkaline borosilicate glass systems are highly valued for their chemical resistance and thermal stability. These properties make them ideal for manufacturing laboratory glassware, storage containers, and drug delivery systems. The growing emphasis on research and development in life sciences and healthcare has further bolstered the demand for these specialized glass products.
The automotive industry has shown increasing interest in alkaline borosilicate glass systems for advanced windshield and window applications. The chemical reactivity of these glasses allows for the integration of smart features, such as heads-up displays and self-tinting capabilities, enhancing both safety and comfort for drivers and passengers.
The renewable energy sector, particularly solar energy, has emerged as a promising market for alkaline borosilicate glass systems. These glasses are used in the production of high-efficiency solar panels and concentrated solar power systems, contributing to the global shift towards sustainable energy sources.
In the construction industry, alkaline borosilicate glass systems are gaining traction for their use in energy-efficient building materials. The chemical properties of these glasses allow for the development of smart windows and insulating glass units that can significantly reduce energy consumption in buildings.
The food and beverage industry has also recognized the benefits of alkaline borosilicate glass systems, particularly in packaging applications. The chemical inertness of these glasses ensures that the contents remain uncontaminated, making them ideal for storing and preserving food and beverages.
As industries continue to seek innovative materials with enhanced properties, the market demand for alkaline borosilicate glass systems is expected to grow. The versatility and unique chemical reactivity of these glasses position them as key components in addressing various technological and environmental challenges across multiple sectors.
The electronics industry has also embraced alkaline borosilicate glass systems, particularly in the production of display panels and touchscreens. The chemical reactivity of these glasses allows for the creation of high-quality, durable surfaces that can withstand frequent use and environmental factors. As the demand for smart devices continues to rise, the market for specialized glass components is expected to expand correspondingly.
In the pharmaceutical and laboratory equipment sector, alkaline borosilicate glass systems are highly valued for their chemical resistance and thermal stability. These properties make them ideal for manufacturing laboratory glassware, storage containers, and drug delivery systems. The growing emphasis on research and development in life sciences and healthcare has further bolstered the demand for these specialized glass products.
The automotive industry has shown increasing interest in alkaline borosilicate glass systems for advanced windshield and window applications. The chemical reactivity of these glasses allows for the integration of smart features, such as heads-up displays and self-tinting capabilities, enhancing both safety and comfort for drivers and passengers.
The renewable energy sector, particularly solar energy, has emerged as a promising market for alkaline borosilicate glass systems. These glasses are used in the production of high-efficiency solar panels and concentrated solar power systems, contributing to the global shift towards sustainable energy sources.
In the construction industry, alkaline borosilicate glass systems are gaining traction for their use in energy-efficient building materials. The chemical properties of these glasses allow for the development of smart windows and insulating glass units that can significantly reduce energy consumption in buildings.
The food and beverage industry has also recognized the benefits of alkaline borosilicate glass systems, particularly in packaging applications. The chemical inertness of these glasses ensures that the contents remain uncontaminated, making them ideal for storing and preserving food and beverages.
As industries continue to seek innovative materials with enhanced properties, the market demand for alkaline borosilicate glass systems is expected to grow. The versatility and unique chemical reactivity of these glasses position them as key components in addressing various technological and environmental challenges across multiple sectors.
Current Challenges in Glass Reactivity
The field of chemical reactivity in alkaline borosilicate glass systems faces several significant challenges that hinder progress and limit practical applications. One of the primary issues is the complexity of glass dissolution mechanisms in alkaline environments. The interaction between glass components and alkaline solutions involves multiple simultaneous processes, making it difficult to isolate and study individual factors contributing to glass degradation.
Another major challenge is the long-term prediction of glass behavior under varying environmental conditions. While short-term laboratory experiments provide valuable insights, extrapolating these results to predict glass performance over decades or centuries remains problematic. This is particularly crucial for applications such as nuclear waste immobilization, where glass stability must be ensured for extended periods.
The heterogeneity of glass compositions further complicates research efforts. Borosilicate glasses can have widely varying compositions depending on their intended use, and each composition may exhibit unique reactivity patterns. This diversity makes it challenging to develop universal models or theories that can accurately describe the behavior of all alkaline borosilicate glass systems.
Researchers also face difficulties in accurately measuring and characterizing the altered layers that form on glass surfaces during alkaline attack. These layers can be extremely thin and compositionally complex, requiring advanced analytical techniques that may not be readily available or may have limitations in resolution or sensitivity.
The role of minor components in glass reactivity presents another significant challenge. Trace elements or impurities can have disproportionate effects on glass durability and reactivity, but their influences are often poorly understood and difficult to quantify. This knowledge gap hampers efforts to optimize glass compositions for specific applications or environmental conditions.
Furthermore, the interplay between chemical and physical processes during glass alteration remains a subject of ongoing debate. Phenomena such as ion exchange, hydrolysis, and precipitation occur simultaneously, and their relative contributions to overall glass degradation can vary depending on environmental factors and glass composition. Developing comprehensive models that account for these interconnected processes is a formidable task.
Lastly, the translation of laboratory findings to real-world applications poses significant challenges. Scaling up experimental results and accounting for the complex, dynamic conditions found in practical settings require careful consideration and often lead to unexpected outcomes. This gap between laboratory research and practical implementation continues to be a major hurdle in advancing the field of alkaline borosilicate glass reactivity.
Another major challenge is the long-term prediction of glass behavior under varying environmental conditions. While short-term laboratory experiments provide valuable insights, extrapolating these results to predict glass performance over decades or centuries remains problematic. This is particularly crucial for applications such as nuclear waste immobilization, where glass stability must be ensured for extended periods.
The heterogeneity of glass compositions further complicates research efforts. Borosilicate glasses can have widely varying compositions depending on their intended use, and each composition may exhibit unique reactivity patterns. This diversity makes it challenging to develop universal models or theories that can accurately describe the behavior of all alkaline borosilicate glass systems.
Researchers also face difficulties in accurately measuring and characterizing the altered layers that form on glass surfaces during alkaline attack. These layers can be extremely thin and compositionally complex, requiring advanced analytical techniques that may not be readily available or may have limitations in resolution or sensitivity.
The role of minor components in glass reactivity presents another significant challenge. Trace elements or impurities can have disproportionate effects on glass durability and reactivity, but their influences are often poorly understood and difficult to quantify. This knowledge gap hampers efforts to optimize glass compositions for specific applications or environmental conditions.
Furthermore, the interplay between chemical and physical processes during glass alteration remains a subject of ongoing debate. Phenomena such as ion exchange, hydrolysis, and precipitation occur simultaneously, and their relative contributions to overall glass degradation can vary depending on environmental factors and glass composition. Developing comprehensive models that account for these interconnected processes is a formidable task.
Lastly, the translation of laboratory findings to real-world applications poses significant challenges. Scaling up experimental results and accounting for the complex, dynamic conditions found in practical settings require careful consideration and often lead to unexpected outcomes. This gap between laboratory research and practical implementation continues to be a major hurdle in advancing the field of alkaline borosilicate glass reactivity.
Existing Solutions for Reactivity Control
01 Composition and properties of alkaline borosilicate glass
Alkaline borosilicate glass systems are characterized by their unique composition, which typically includes silica, boron oxide, and alkali metal oxides. This composition results in specific chemical and physical properties, such as high chemical durability, low thermal expansion, and resistance to thermal shock. The alkaline component influences the glass network structure and reactivity.- Composition and properties of alkaline borosilicate glass: Alkaline borosilicate glass systems are characterized by their unique composition, typically containing alkali oxides, boron oxide, and silica. These glasses exhibit specific chemical and physical properties, including high chemical durability, low thermal expansion, and resistance to thermal shock. The alkaline component influences the glass network structure and reactivity.
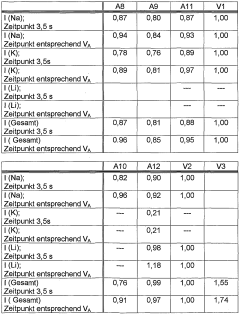
- Chemical reactivity with aqueous solutions: Alkaline borosilicate glasses show varying degrees of reactivity when exposed to aqueous solutions. The chemical durability and dissolution behavior depend on factors such as pH, temperature, and solution composition. In alkaline environments, these glasses may undergo selective leaching of certain components, affecting their surface properties and long-term stability.
- Surface modification and functionalization: The surface of alkaline borosilicate glasses can be modified to enhance specific properties or functionalities. Various techniques, including chemical treatments, coatings, and ion exchange processes, can be employed to alter the surface chemistry and reactivity. These modifications can improve characteristics such as biocompatibility, catalytic activity, or resistance to specific chemical environments.
- Application in nuclear waste immobilization: Alkaline borosilicate glass systems are widely used for nuclear waste immobilization due to their chemical stability and ability to incorporate various radioactive elements. The chemical reactivity of these glasses in repository conditions is crucial for long-term waste containment. Research focuses on understanding and improving the glass performance under different geological and chemical environments.
- Corrosion resistance and durability: The corrosion resistance of alkaline borosilicate glasses is a key factor in their chemical reactivity. Studies investigate the mechanisms of glass corrosion, including ion exchange, hydrolysis, and precipitation of secondary phases. Understanding these processes helps in developing more durable glass compositions for various applications, from laboratory glassware to industrial equipment exposed to harsh chemical environments.
02 Chemical reactivity with aqueous solutions
Alkaline borosilicate glasses exhibit varying degrees of chemical reactivity when exposed to aqueous solutions. The reactivity is influenced by factors such as pH, temperature, and solution composition. In alkaline environments, these glasses may undergo selective leaching of certain components, leading to the formation of surface layers with altered properties.Expand Specific Solutions03 Surface modification and functionalization
The surface of alkaline borosilicate glass can be modified to enhance or alter its chemical reactivity. Various techniques, such as ion exchange, coating, or chemical treatment, can be employed to functionalize the glass surface. These modifications can improve properties like biocompatibility, catalytic activity, or specific chemical resistance.Expand Specific Solutions04 Corrosion resistance and durability
Alkaline borosilicate glasses are known for their high corrosion resistance in various chemical environments. The presence of boron in the glass network contributes to improved chemical durability. However, the extent of corrosion resistance can vary depending on the specific glass composition and the corrosive medium. Understanding these interactions is crucial for applications in harsh chemical environments.Expand Specific Solutions05 Applications in chemical and pharmaceutical industries
The chemical reactivity properties of alkaline borosilicate glass systems make them suitable for various applications in chemical and pharmaceutical industries. These include laboratory glassware, chemical storage containers, and pharmaceutical packaging. The glass composition can be tailored to meet specific requirements for chemical compatibility, drug stability, and sterilization processes.Expand Specific Solutions
Key Players in Borosilicate Glass Industry
The chemical reactivity in alkaline borosilicate glass systems is a mature field with significant market potential, driven by applications in pharmaceuticals, electronics, and specialty materials. The industry is in a growth phase, with a global market size estimated to exceed $5 billion. Key players like SCHOTT AG, Corning, Inc., and Nippon Electric Glass Co., Ltd. have established strong positions through decades of research and development. These companies leverage their expertise in glass formulation and manufacturing to create high-performance products for diverse applications. The competitive landscape is characterized by ongoing innovation in glass composition and processing techniques, with a focus on enhancing chemical durability and tailoring properties for specific end-uses.
SCHOTT AG
Technical Solution: SCHOTT AG has developed advanced alkaline borosilicate glass systems with enhanced chemical durability. Their DURAN® borosilicate glass exhibits exceptional resistance to chemical attack in alkaline environments[1]. The company utilizes a proprietary melting process that ensures homogeneous glass composition, minimizing the risk of devitrification and improving overall chemical stability[2]. SCHOTT's alkaline-resistant borosilicate glasses are engineered with optimized B2O3/SiO2 ratios and controlled alkali content, resulting in a dense glass network that resists corrosion even in highly alkaline conditions[3]. The company has also implemented surface treatment techniques, such as ion exchange, to further enhance the chemical durability of their borosilicate glass products[4].
Strengths: Superior chemical resistance in alkaline environments, homogeneous glass composition, and advanced surface treatment techniques. Weaknesses: Potentially higher production costs due to specialized manufacturing processes and limited flexibility in glass composition adjustments.
Corning, Inc.
Technical Solution: Corning has developed a range of alkaline-resistant borosilicate glass compositions tailored for various applications. Their Valor® Glass technology incorporates a unique aluminosilicate composition that enhances chemical durability in alkaline environments[1]. Corning's approach involves precise control of glass network formers and modifiers, optimizing the ratio of bridging to non-bridging oxygen atoms to improve resistance to alkaline attack[2]. The company has also implemented innovative surface treatments, such as ion-exchange strengthening, which creates a compressive stress layer that further enhances chemical resistance[3]. Corning's research has focused on understanding and mitigating the mechanisms of glass dissolution in alkaline media, leading to the development of glass compositions with reduced leaching rates and improved long-term stability[4].
Strengths: Innovative glass compositions with enhanced alkaline resistance, advanced surface treatment technologies, and extensive research into glass dissolution mechanisms. Weaknesses: Potential limitations in extreme alkaline environments and higher production costs for specialized compositions.
Innovative Approaches to Glass Stability
Borosilicate glass
PatentInactiveEP0767763A2
Innovation
- A borosilicate glass composition with specific thermal, electrical, and chemical properties, including SiO2, ZrO2, Na2O, K2O, CaO, MgO, BaO, and Al2O3, optimized for stable electrical conductivity and viscosity, allowing full-electrical melting in cold-top furnaces with reduced gas release and no toxic heavy metal oxides, ensuring stable melting and high chemical resistance.
Borosilicate glass with high chemical resistance and use thereof
PatentWO2002008134A1
Innovation
- A borosilicate glass composition with specific weight percentages of SiO2, B2O3, Al2O3, alkali metal oxides, and other components, optimized to achieve low processing temperatures, high chemical resistance, and a thermal expansion coefficient close to that of metals like Fe-Ni-Co alloys, while minimizing alkali evaporation and crystallization risks.
Environmental Impact of Glass Production
The production of glass, particularly alkaline borosilicate glass systems, has significant environmental implications that warrant careful consideration. The manufacturing process involves high-temperature melting of raw materials, which consumes substantial amounts of energy and contributes to greenhouse gas emissions. Fossil fuels are often used to power furnaces, leading to the release of carbon dioxide and other pollutants into the atmosphere. Additionally, the extraction and processing of raw materials for glass production, such as silica sand, soda ash, and boron compounds, can have detrimental effects on local ecosystems and biodiversity.
The chemical reactivity of alkaline borosilicate glass systems during production also raises environmental concerns. The release of volatile compounds, including boron and alkali oxides, can contribute to air pollution and potentially impact human health in surrounding areas. Furthermore, the disposal of waste materials generated during the manufacturing process, such as cullet and rejected batches, requires proper management to prevent soil and water contamination.
Water usage in glass production is another critical environmental factor. The cooling processes and cleaning of equipment consume significant amounts of water, potentially straining local water resources. Effluents from these processes may contain dissolved metals and other contaminants, necessitating proper treatment before discharge to prevent water pollution.
The durability and recyclability of borosilicate glass products offer some environmental benefits. The long lifespan of these products reduces the need for frequent replacements, thereby minimizing waste generation. Moreover, the recyclability of glass helps conserve raw materials and energy, as recycled glass can be remelted and reformed multiple times without significant loss of quality.
However, the recycling process itself presents challenges. The chemical composition of borosilicate glass differs from common soda-lime glass, requiring separate recycling streams to maintain product quality. This segregation can complicate recycling efforts and potentially lead to increased landfill disposal if proper recycling infrastructure is not in place.
Efforts to mitigate the environmental impact of glass production focus on improving energy efficiency, reducing emissions, and developing more sustainable manufacturing processes. These include the use of electric melting technologies, implementation of heat recovery systems, and exploration of alternative raw materials. Additionally, research into novel glass compositions that require lower melting temperatures or incorporate higher percentages of recycled content is ongoing, aiming to reduce the overall environmental footprint of glass production.
The chemical reactivity of alkaline borosilicate glass systems during production also raises environmental concerns. The release of volatile compounds, including boron and alkali oxides, can contribute to air pollution and potentially impact human health in surrounding areas. Furthermore, the disposal of waste materials generated during the manufacturing process, such as cullet and rejected batches, requires proper management to prevent soil and water contamination.
Water usage in glass production is another critical environmental factor. The cooling processes and cleaning of equipment consume significant amounts of water, potentially straining local water resources. Effluents from these processes may contain dissolved metals and other contaminants, necessitating proper treatment before discharge to prevent water pollution.
The durability and recyclability of borosilicate glass products offer some environmental benefits. The long lifespan of these products reduces the need for frequent replacements, thereby minimizing waste generation. Moreover, the recyclability of glass helps conserve raw materials and energy, as recycled glass can be remelted and reformed multiple times without significant loss of quality.
However, the recycling process itself presents challenges. The chemical composition of borosilicate glass differs from common soda-lime glass, requiring separate recycling streams to maintain product quality. This segregation can complicate recycling efforts and potentially lead to increased landfill disposal if proper recycling infrastructure is not in place.
Efforts to mitigate the environmental impact of glass production focus on improving energy efficiency, reducing emissions, and developing more sustainable manufacturing processes. These include the use of electric melting technologies, implementation of heat recovery systems, and exploration of alternative raw materials. Additionally, research into novel glass compositions that require lower melting temperatures or incorporate higher percentages of recycled content is ongoing, aiming to reduce the overall environmental footprint of glass production.
Regulatory Framework for Glass Materials
The regulatory framework for glass materials, particularly in the context of chemical reactivity in alkaline borosilicate glass systems, is a complex and evolving landscape. Governments and international organizations have established various standards and guidelines to ensure the safety and quality of glass materials used in different applications.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating glass materials used in food packaging and pharmaceutical containers. The FDA's Code of Federal Regulations (CFR) Title 21, Part 177 outlines specific requirements for glass and ceramic materials that come into contact with food. These regulations focus on the chemical composition and potential leaching of harmful substances from glass materials.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which affects the production and use of glass materials. Under REACH, manufacturers must register chemical substances used in glass production and demonstrate their safe use. Additionally, the EU's Restriction of Hazardous Substances (RoHS) Directive limits the use of certain hazardous substances in electrical and electronic equipment, including glass components.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, have developed specific standards for glass materials. ISO 719 and ISO 720, for example, provide methods for testing the hydrolytic resistance of glass grains, which is particularly relevant for borosilicate glass systems. ASTM C1285 outlines the standard test methods for determining chemical durability of nuclear, hazardous, and mixed waste glasses and multiphase glass ceramics.
In the context of nuclear waste management, where alkaline borosilicate glass systems are commonly used for vitrification, regulatory bodies such as the Nuclear Regulatory Commission (NRC) in the United States and the International Atomic Energy Agency (IAEA) have established guidelines for the use of glass materials in waste immobilization. These regulations focus on long-term stability, chemical durability, and radiation resistance of glass matrices.
Environmental regulations also impact the production and disposal of glass materials. The Environmental Protection Agency (EPA) in the United States regulates the disposal of glass waste and emissions from glass manufacturing facilities under the Resource Conservation and Recovery Act (RCRA) and the Clean Air Act, respectively.
As research in alkaline borosilicate glass systems advances, regulatory frameworks are expected to evolve to address new findings on chemical reactivity and potential environmental impacts. Manufacturers and researchers must stay informed about these regulatory changes to ensure compliance and promote the development of safer, more durable glass materials.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating glass materials used in food packaging and pharmaceutical containers. The FDA's Code of Federal Regulations (CFR) Title 21, Part 177 outlines specific requirements for glass and ceramic materials that come into contact with food. These regulations focus on the chemical composition and potential leaching of harmful substances from glass materials.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which affects the production and use of glass materials. Under REACH, manufacturers must register chemical substances used in glass production and demonstrate their safe use. Additionally, the EU's Restriction of Hazardous Substances (RoHS) Directive limits the use of certain hazardous substances in electrical and electronic equipment, including glass components.
International standards organizations, such as the International Organization for Standardization (ISO) and ASTM International, have developed specific standards for glass materials. ISO 719 and ISO 720, for example, provide methods for testing the hydrolytic resistance of glass grains, which is particularly relevant for borosilicate glass systems. ASTM C1285 outlines the standard test methods for determining chemical durability of nuclear, hazardous, and mixed waste glasses and multiphase glass ceramics.
In the context of nuclear waste management, where alkaline borosilicate glass systems are commonly used for vitrification, regulatory bodies such as the Nuclear Regulatory Commission (NRC) in the United States and the International Atomic Energy Agency (IAEA) have established guidelines for the use of glass materials in waste immobilization. These regulations focus on long-term stability, chemical durability, and radiation resistance of glass matrices.
Environmental regulations also impact the production and disposal of glass materials. The Environmental Protection Agency (EPA) in the United States regulates the disposal of glass waste and emissions from glass manufacturing facilities under the Resource Conservation and Recovery Act (RCRA) and the Clean Air Act, respectively.
As research in alkaline borosilicate glass systems advances, regulatory frameworks are expected to evolve to address new findings on chemical reactivity and potential environmental impacts. Manufacturers and researchers must stay informed about these regulatory changes to ensure compliance and promote the development of safer, more durable glass materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!