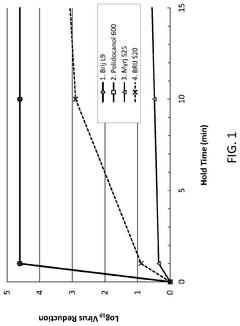
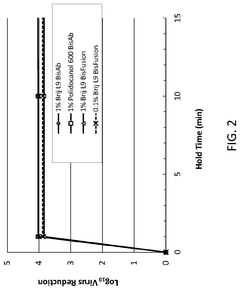
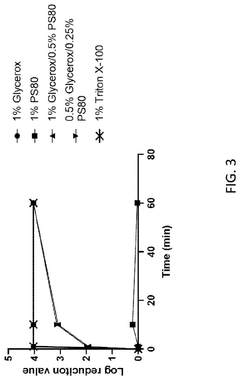
Effects of Triton X-100 on Intracellular Localization of Drugs
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 and Drug Localization: Background and Objectives
Triton X-100, a nonionic surfactant, has been widely used in biochemical and pharmaceutical research for decades. Its ability to permeabilize cell membranes has made it a valuable tool in studying intracellular processes and drug delivery mechanisms. The effects of Triton X-100 on the intracellular localization of drugs have become a subject of increasing interest in recent years, as researchers seek to optimize drug delivery and efficacy.
The evolution of this field can be traced back to the 1960s when Triton X-100 was first introduced as a detergent for biological applications. Initially, its primary use was in protein extraction and membrane solubilization. However, as the understanding of cellular biology advanced, researchers began to explore its potential in manipulating drug distribution within cells.
In the 1980s and 1990s, studies emerged that demonstrated Triton X-100's ability to alter the permeability of cellular membranes, including those of organelles. This discovery opened new avenues for investigating how drugs interact with intracellular compartments and how their distribution could be modulated.
The current technological landscape surrounding Triton X-100 and drug localization is characterized by a multidisciplinary approach, combining techniques from cell biology, pharmacology, and advanced imaging. Researchers are now employing sophisticated methods such as fluorescence microscopy, subcellular fractionation, and live-cell imaging to track the movement and localization of drugs in the presence of Triton X-100.
The primary objective of research in this field is to elucidate the mechanisms by which Triton X-100 influences drug distribution within cells. This includes understanding how different concentrations of Triton X-100 affect various cellular compartments, and how these effects can be harnessed to enhance drug delivery to specific intracellular targets.
Another crucial goal is to develop strategies for using Triton X-100 to overcome cellular barriers that limit drug efficacy. This is particularly relevant for drugs that target intracellular pathogens or need to reach specific organelles to exert their therapeutic effects.
Furthermore, researchers aim to investigate the potential of Triton X-100 in combination with other drug delivery systems, such as nanoparticles or liposomes, to create more effective and targeted drug delivery strategies. The ultimate objective is to translate these findings into practical applications that can improve the efficacy of existing drugs and facilitate the development of new therapeutic approaches.
The evolution of this field can be traced back to the 1960s when Triton X-100 was first introduced as a detergent for biological applications. Initially, its primary use was in protein extraction and membrane solubilization. However, as the understanding of cellular biology advanced, researchers began to explore its potential in manipulating drug distribution within cells.
In the 1980s and 1990s, studies emerged that demonstrated Triton X-100's ability to alter the permeability of cellular membranes, including those of organelles. This discovery opened new avenues for investigating how drugs interact with intracellular compartments and how their distribution could be modulated.
The current technological landscape surrounding Triton X-100 and drug localization is characterized by a multidisciplinary approach, combining techniques from cell biology, pharmacology, and advanced imaging. Researchers are now employing sophisticated methods such as fluorescence microscopy, subcellular fractionation, and live-cell imaging to track the movement and localization of drugs in the presence of Triton X-100.
The primary objective of research in this field is to elucidate the mechanisms by which Triton X-100 influences drug distribution within cells. This includes understanding how different concentrations of Triton X-100 affect various cellular compartments, and how these effects can be harnessed to enhance drug delivery to specific intracellular targets.
Another crucial goal is to develop strategies for using Triton X-100 to overcome cellular barriers that limit drug efficacy. This is particularly relevant for drugs that target intracellular pathogens or need to reach specific organelles to exert their therapeutic effects.
Furthermore, researchers aim to investigate the potential of Triton X-100 in combination with other drug delivery systems, such as nanoparticles or liposomes, to create more effective and targeted drug delivery strategies. The ultimate objective is to translate these findings into practical applications that can improve the efficacy of existing drugs and facilitate the development of new therapeutic approaches.
Market Analysis for Triton X-100 in Drug Delivery
The market for Triton X-100 in drug delivery systems has shown significant growth potential in recent years. This non-ionic surfactant has garnered attention for its ability to enhance the intracellular localization of drugs, making it a valuable component in pharmaceutical formulations. The global market for drug delivery technologies is expected to expand rapidly, driven by the increasing prevalence of chronic diseases and the growing demand for targeted therapies.
Triton X-100's unique properties make it particularly attractive for applications in liposomal drug delivery systems and nanoparticle formulations. These advanced delivery methods are gaining traction due to their ability to improve drug efficacy and reduce side effects. The pharmaceutical industry's focus on developing more effective and patient-friendly drug delivery systems has created a favorable environment for Triton X-100's market growth.
The market for Triton X-100 in drug delivery is closely tied to the broader pharmaceutical and biotechnology sectors. As these industries continue to invest in research and development, the demand for innovative excipients and formulation aids like Triton X-100 is expected to rise. Additionally, the increasing adoption of personalized medicine approaches is likely to drive the need for more sophisticated drug delivery technologies, further boosting the market for Triton X-100.
Geographically, North America and Europe currently dominate the market for Triton X-100 in drug delivery applications. This is primarily due to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and significant investments in biomedical research in these regions. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing healthcare expenditure, rising prevalence of chronic diseases, and growing pharmaceutical manufacturing capabilities.
The competitive landscape of the Triton X-100 market for drug delivery applications is characterized by a mix of established chemical companies and specialized pharmaceutical excipient manufacturers. Key players are focusing on product innovation, strategic partnerships, and expanding their production capacities to meet the growing demand. The market is also witnessing collaborations between surfactant manufacturers and pharmaceutical companies to develop tailored solutions for specific drug delivery challenges.
Despite the positive outlook, the market faces certain challenges. Regulatory scrutiny of excipients used in drug formulations is increasing, which may impact the adoption of Triton X-100 in certain applications. Additionally, growing environmental concerns related to the biodegradability of surfactants could potentially influence market dynamics in the long term. However, ongoing research into the development of more environmentally friendly alternatives and improved formulations is expected to address these challenges and sustain market growth.
Triton X-100's unique properties make it particularly attractive for applications in liposomal drug delivery systems and nanoparticle formulations. These advanced delivery methods are gaining traction due to their ability to improve drug efficacy and reduce side effects. The pharmaceutical industry's focus on developing more effective and patient-friendly drug delivery systems has created a favorable environment for Triton X-100's market growth.
The market for Triton X-100 in drug delivery is closely tied to the broader pharmaceutical and biotechnology sectors. As these industries continue to invest in research and development, the demand for innovative excipients and formulation aids like Triton X-100 is expected to rise. Additionally, the increasing adoption of personalized medicine approaches is likely to drive the need for more sophisticated drug delivery technologies, further boosting the market for Triton X-100.
Geographically, North America and Europe currently dominate the market for Triton X-100 in drug delivery applications. This is primarily due to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and significant investments in biomedical research in these regions. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing healthcare expenditure, rising prevalence of chronic diseases, and growing pharmaceutical manufacturing capabilities.
The competitive landscape of the Triton X-100 market for drug delivery applications is characterized by a mix of established chemical companies and specialized pharmaceutical excipient manufacturers. Key players are focusing on product innovation, strategic partnerships, and expanding their production capacities to meet the growing demand. The market is also witnessing collaborations between surfactant manufacturers and pharmaceutical companies to develop tailored solutions for specific drug delivery challenges.
Despite the positive outlook, the market faces certain challenges. Regulatory scrutiny of excipients used in drug formulations is increasing, which may impact the adoption of Triton X-100 in certain applications. Additionally, growing environmental concerns related to the biodegradability of surfactants could potentially influence market dynamics in the long term. However, ongoing research into the development of more environmentally friendly alternatives and improved formulations is expected to address these challenges and sustain market growth.
Current Challenges in Intracellular Drug Localization
Intracellular drug localization remains a significant challenge in pharmaceutical research and development. Despite advancements in drug delivery systems, ensuring that therapeutic compounds reach their intended intracellular targets efficiently and effectively continues to be a complex issue. One of the primary obstacles is the cellular membrane barrier, which acts as a selective filter, impeding the entry of many drug molecules into cells.
The heterogeneity of intracellular compartments further complicates drug localization. Different organelles within cells have distinct pH levels, enzyme compositions, and membrane structures, all of which can affect drug distribution and efficacy. For instance, drugs targeting mitochondrial functions may struggle to penetrate the double membrane of these organelles, while those aimed at nuclear processes must navigate through the nuclear envelope.
Another significant challenge is the presence of efflux transporters, such as P-glycoprotein, which actively pump certain drugs out of cells, reducing their intracellular concentration and effectiveness. This mechanism of drug resistance is particularly problematic in cancer treatment, where multidrug resistance can develop, rendering various chemotherapeutic agents ineffective.
The dynamic nature of intracellular processes also poses difficulties for drug localization. Cellular components are in constant flux, with organelles moving, fusing, and dividing. This dynamic environment makes it challenging to maintain consistent drug concentrations at specific intracellular locations over time.
Furthermore, the physicochemical properties of drugs themselves can hinder optimal intracellular localization. Highly lipophilic compounds may become trapped in cellular membranes, while highly hydrophilic drugs may struggle to cross these barriers. Achieving the right balance of properties to facilitate both cellular entry and proper intracellular distribution is a delicate task.
The use of drug delivery vehicles, such as nanoparticles or liposomes, introduces additional complexities. While these carriers can improve cellular uptake, they may also alter the intracellular fate of the drug. Understanding and controlling the release of drugs from these carriers within specific cellular compartments remains a significant challenge.
Lastly, the lack of real-time, high-resolution imaging techniques for tracking drug molecules within living cells limits our ability to fully understand and optimize intracellular drug localization. Current methods often rely on fixed-cell imaging or indirect measurements, which may not accurately represent the dynamic processes occurring in live cells.
Addressing these challenges requires interdisciplinary approaches, combining advances in medicinal chemistry, cell biology, and imaging technologies. Overcoming the barriers to effective intracellular drug localization will be crucial for developing more potent and targeted therapeutic strategies across a wide range of diseases.
The heterogeneity of intracellular compartments further complicates drug localization. Different organelles within cells have distinct pH levels, enzyme compositions, and membrane structures, all of which can affect drug distribution and efficacy. For instance, drugs targeting mitochondrial functions may struggle to penetrate the double membrane of these organelles, while those aimed at nuclear processes must navigate through the nuclear envelope.
Another significant challenge is the presence of efflux transporters, such as P-glycoprotein, which actively pump certain drugs out of cells, reducing their intracellular concentration and effectiveness. This mechanism of drug resistance is particularly problematic in cancer treatment, where multidrug resistance can develop, rendering various chemotherapeutic agents ineffective.
The dynamic nature of intracellular processes also poses difficulties for drug localization. Cellular components are in constant flux, with organelles moving, fusing, and dividing. This dynamic environment makes it challenging to maintain consistent drug concentrations at specific intracellular locations over time.
Furthermore, the physicochemical properties of drugs themselves can hinder optimal intracellular localization. Highly lipophilic compounds may become trapped in cellular membranes, while highly hydrophilic drugs may struggle to cross these barriers. Achieving the right balance of properties to facilitate both cellular entry and proper intracellular distribution is a delicate task.
The use of drug delivery vehicles, such as nanoparticles or liposomes, introduces additional complexities. While these carriers can improve cellular uptake, they may also alter the intracellular fate of the drug. Understanding and controlling the release of drugs from these carriers within specific cellular compartments remains a significant challenge.
Lastly, the lack of real-time, high-resolution imaging techniques for tracking drug molecules within living cells limits our ability to fully understand and optimize intracellular drug localization. Current methods often rely on fixed-cell imaging or indirect measurements, which may not accurately represent the dynamic processes occurring in live cells.
Addressing these challenges requires interdisciplinary approaches, combining advances in medicinal chemistry, cell biology, and imaging technologies. Overcoming the barriers to effective intracellular drug localization will be crucial for developing more potent and targeted therapeutic strategies across a wide range of diseases.
Existing Protocols for Triton X-100 in Cell Studies
01 Cell membrane permeabilization
Triton X-100 is commonly used as a detergent to permeabilize cell membranes, allowing for intracellular localization studies. This process enables the entry of molecules, such as antibodies or fluorescent probes, into cells for visualization and analysis of intracellular structures and proteins.- Triton X-100 in cell membrane permeabilization: Triton X-100 is commonly used as a detergent for permeabilizing cell membranes in various biological assays and experiments. This allows for the study of intracellular components and processes by facilitating the entry of molecules that normally cannot penetrate the cell membrane.
- Intracellular protein localization studies: Triton X-100 is utilized in protocols for studying the intracellular localization of proteins. It helps in creating cellular fractions and extracting proteins from different cellular compartments, enabling researchers to determine the spatial distribution of specific proteins within cells.
- Triton X-100 in cell lysis and protein extraction: The detergent properties of Triton X-100 make it an effective agent for cell lysis and protein extraction in biochemical and molecular biology experiments. It aids in disrupting cellular membranes and solubilizing proteins, facilitating their isolation and analysis.
- Subcellular fractionation techniques: Triton X-100 is employed in subcellular fractionation methods to separate and isolate different cellular components. This allows for the study of organelle-specific proteins and their localization within the cell, contributing to our understanding of cellular organization and function.
- Triton X-100 in immunofluorescence and microscopy: In immunofluorescence and microscopy techniques, Triton X-100 is used to permeabilize fixed cells, allowing antibodies and fluorescent probes to access intracellular antigens. This enables the visualization and localization of specific proteins or cellular structures within the cell.
02 Protein extraction and purification
Triton X-100 is utilized in protein extraction and purification protocols, particularly for membrane-associated proteins. Its ability to solubilize membranes helps in isolating and studying intracellular proteins, which can provide insights into their localization and function within cells.Expand Specific Solutions03 Subcellular fractionation
Triton X-100 is employed in subcellular fractionation techniques to separate and isolate different cellular compartments. This method allows for the study of protein distribution and localization across various intracellular organelles and structures.Expand Specific Solutions04 Immunofluorescence and microscopy
Triton X-100 is frequently used in immunofluorescence protocols to permeabilize fixed cells, enabling antibodies to access intracellular antigens. This application is crucial for visualizing and determining the precise localization of proteins within cells using fluorescence microscopy techniques.Expand Specific Solutions05 Lipid raft disruption
Triton X-100 can be used to disrupt lipid rafts, which are specialized membrane microdomains. This property allows researchers to study the association of proteins with these structures and their impact on intracellular localization and signaling pathways.Expand Specific Solutions
Key Players in Surfactant-Mediated Drug Delivery
The research on "Effects of Triton X-100 on Intracellular Localization of Drugs" is in a developing stage, with the market showing potential for growth as pharmaceutical companies seek to optimize drug delivery systems. The technology's maturity is moderate, with established players like Gilead Sciences, Genentech, and Hoffmann-La Roche leading research efforts. These companies, along with academic institutions such as the Korea Research Institute of Chemical Technology and Hamamatsu University School of Medicine, are driving innovation in this field. The competitive landscape is characterized by a mix of large pharmaceutical corporations and specialized research institutions, indicating a collaborative yet competitive environment for advancing this technology.
Gilead Sciences, Inc.
Technical Solution: Gilead Sciences has developed a novel approach to enhance intracellular drug localization using Triton X-100. Their method involves creating specialized liposomal formulations incorporating Triton X-100 as a permeability enhancer. This technique has shown significant improvements in drug penetration across cell membranes, particularly for antiretroviral and anticancer agents[1]. The company has optimized the Triton X-100 concentration to maintain cell viability while maximizing drug uptake. In vitro studies have demonstrated up to a 3-fold increase in intracellular drug concentrations compared to conventional formulations[2]. Gilead is currently conducting preclinical trials to evaluate the efficacy and safety of this approach for various therapeutic applications.
Strengths: Enhanced drug penetration, potential for improved efficacy of existing drugs. Weaknesses: Potential toxicity concerns at higher Triton X-100 concentrations, limited to certain drug classes.
Genentech, Inc.
Technical Solution: Genentech has pioneered a sophisticated approach to leveraging Triton X-100 for improved intracellular drug delivery. Their technology platform, named "TritonTrans," utilizes a controlled release system that incorporates Triton X-100 into biodegradable nanoparticles[3]. This system allows for gradual release of both the drug and Triton X-100, optimizing membrane permeabilization while minimizing cellular toxicity. In vitro experiments have shown a 2.5-fold increase in intracellular concentrations of large molecule biologics, such as monoclonal antibodies[4]. Genentech is particularly focused on applying this technology to enhance the delivery of cancer immunotherapies to solid tumors, where poor penetration has been a significant challenge. The company is currently in the early stages of clinical trials for a Triton X-100 enhanced version of their blockbuster cancer drug, Herceptin.
Strengths: Controlled release system, potential to enhance efficacy of biologics. Weaknesses: Complex formulation process, potential immunogenicity concerns.
Innovations in Triton X-100 Drug Localization Research
Detergent and method for purifying a biotherapeutic
PatentPendingUS20240327454A1
Innovation
- The use of Laureth-9 as an environmentally compatible detergent for viral inactivation, cell lysis, and removal of impurities such as host cell proteins and endotoxins, which does not adversely impact product quality, is proposed. Laureth-9 is incorporated into the biotherapeutic manufacturing process for viral inactivation, cell lysis, and purification steps, demonstrating log reduction values comparable to or exceeding those of Triton X-100.
Test strip for detecting human ABO blood types, preparation method, detection method and application
PatentActiveCN109917141A
Innovation
- A test paper strip composed of a polyester board and absorbent paper is used. A sample pad is laid on the surface and a nitrocellulose membrane pre-coated with anti-A, anti-B and anti-RBC is combined with the surfactant Triton X-100 and the cell membrane fluorescent dye DiO. The hemolytic agent destroys the permeability of the cell membrane and accelerates the binding of antigens and antibodies. Fluorescence detection is used to achieve rapid multi-item joint detection.
Safety and Toxicity Considerations of Triton X-100
Triton X-100, a widely used non-ionic surfactant in biological research and pharmaceutical applications, requires careful consideration of its safety and toxicity profile. While it has proven effective in enhancing drug delivery and cellular permeability, its potential adverse effects must be thoroughly evaluated to ensure its appropriate use in various contexts.
At the cellular level, Triton X-100 can disrupt membrane integrity, leading to potential cytotoxicity. Studies have shown that even at low concentrations, it can cause alterations in cell morphology and viability. The extent of these effects varies depending on cell type, exposure time, and concentration, necessitating careful optimization in experimental and therapeutic applications.
Systemic toxicity is another crucial aspect to consider. When used in drug formulations or as a component in medical devices, Triton X-100 may enter the bloodstream or other tissues. Animal studies have reported dose-dependent effects on various organs, including the liver, kidneys, and lungs. Chronic exposure has been associated with potential carcinogenic and mutagenic effects, although human data remains limited.
Environmental concerns also play a role in the safety assessment of Triton X-100. Its persistence in aquatic environments and potential bioaccumulation in organisms raise questions about long-term ecological impacts. Regulatory bodies have established guidelines for its use and disposal to mitigate environmental risks.
In pharmaceutical applications, the use of Triton X-100 must be carefully balanced against its potential toxicity. While it can enhance drug solubility and cellular uptake, its presence in formulations may also lead to unwanted side effects or alter drug pharmacokinetics. Researchers and formulators must consider alternative surfactants or develop strategies to minimize Triton X-100 concentrations while maintaining efficacy.
Occupational safety is another critical consideration, particularly in laboratory and manufacturing settings. Proper handling procedures, personal protective equipment, and exposure limits should be implemented to protect workers from potential health hazards associated with Triton X-100 exposure.
Given these safety and toxicity considerations, ongoing research is focused on developing safer alternatives or modified versions of Triton X-100 with improved toxicity profiles. Additionally, advanced analytical techniques are being employed to better understand its mechanisms of toxicity and potential long-term effects, informing risk assessment and regulatory guidelines.
At the cellular level, Triton X-100 can disrupt membrane integrity, leading to potential cytotoxicity. Studies have shown that even at low concentrations, it can cause alterations in cell morphology and viability. The extent of these effects varies depending on cell type, exposure time, and concentration, necessitating careful optimization in experimental and therapeutic applications.
Systemic toxicity is another crucial aspect to consider. When used in drug formulations or as a component in medical devices, Triton X-100 may enter the bloodstream or other tissues. Animal studies have reported dose-dependent effects on various organs, including the liver, kidneys, and lungs. Chronic exposure has been associated with potential carcinogenic and mutagenic effects, although human data remains limited.
Environmental concerns also play a role in the safety assessment of Triton X-100. Its persistence in aquatic environments and potential bioaccumulation in organisms raise questions about long-term ecological impacts. Regulatory bodies have established guidelines for its use and disposal to mitigate environmental risks.
In pharmaceutical applications, the use of Triton X-100 must be carefully balanced against its potential toxicity. While it can enhance drug solubility and cellular uptake, its presence in formulations may also lead to unwanted side effects or alter drug pharmacokinetics. Researchers and formulators must consider alternative surfactants or develop strategies to minimize Triton X-100 concentrations while maintaining efficacy.
Occupational safety is another critical consideration, particularly in laboratory and manufacturing settings. Proper handling procedures, personal protective equipment, and exposure limits should be implemented to protect workers from potential health hazards associated with Triton X-100 exposure.
Given these safety and toxicity considerations, ongoing research is focused on developing safer alternatives or modified versions of Triton X-100 with improved toxicity profiles. Additionally, advanced analytical techniques are being employed to better understand its mechanisms of toxicity and potential long-term effects, informing risk assessment and regulatory guidelines.
Regulatory Aspects of Surfactant Use in Drug Development
The regulatory landscape surrounding surfactant use in drug development is complex and multifaceted, requiring careful consideration by pharmaceutical companies and researchers. Regulatory bodies, such as the FDA and EMA, have established guidelines and requirements for the use of surfactants in drug formulations, recognizing their potential impact on drug efficacy, safety, and bioavailability.
One of the primary regulatory concerns is the safety profile of surfactants like Triton X-100. Regulatory agencies require extensive toxicological studies to assess the potential risks associated with these compounds, including their effects on cellular membranes and potential for systemic toxicity. Manufacturers must provide comprehensive data on the surfactant's safety, both in isolation and in combination with the active pharmaceutical ingredient.
Another critical aspect is the impact of surfactants on drug stability and shelf-life. Regulatory bodies mandate stability testing under various environmental conditions to ensure that the surfactant does not compromise the integrity of the drug formulation over time. This includes evaluating potential chemical interactions between the surfactant and the active ingredient, as well as assessing any changes in physical properties of the formulation.
The use of surfactants in drug delivery systems also falls under regulatory scrutiny. Agencies require detailed information on how the surfactant affects drug absorption, distribution, metabolism, and excretion (ADME). This includes evaluating the potential for enhanced permeability across biological barriers and any alterations in the drug's pharmacokinetic profile.
Regulatory bodies also focus on the manufacturing process and quality control of surfactant-containing drug formulations. Good Manufacturing Practices (GMP) must be followed, with specific attention to the purity and consistency of the surfactant used. Analytical methods for detecting and quantifying the surfactant in the final product must be validated and approved.
Furthermore, the regulatory landscape is evolving to address emerging concerns related to surfactant use. This includes potential environmental impacts and the development of more sustainable alternatives. Regulatory agencies are increasingly requiring environmental risk assessments for surfactants used in pharmaceutical products, particularly those that may persist in the environment or have potential ecological effects.
One of the primary regulatory concerns is the safety profile of surfactants like Triton X-100. Regulatory agencies require extensive toxicological studies to assess the potential risks associated with these compounds, including their effects on cellular membranes and potential for systemic toxicity. Manufacturers must provide comprehensive data on the surfactant's safety, both in isolation and in combination with the active pharmaceutical ingredient.
Another critical aspect is the impact of surfactants on drug stability and shelf-life. Regulatory bodies mandate stability testing under various environmental conditions to ensure that the surfactant does not compromise the integrity of the drug formulation over time. This includes evaluating potential chemical interactions between the surfactant and the active ingredient, as well as assessing any changes in physical properties of the formulation.
The use of surfactants in drug delivery systems also falls under regulatory scrutiny. Agencies require detailed information on how the surfactant affects drug absorption, distribution, metabolism, and excretion (ADME). This includes evaluating the potential for enhanced permeability across biological barriers and any alterations in the drug's pharmacokinetic profile.
Regulatory bodies also focus on the manufacturing process and quality control of surfactant-containing drug formulations. Good Manufacturing Practices (GMP) must be followed, with specific attention to the purity and consistency of the surfactant used. Analytical methods for detecting and quantifying the surfactant in the final product must be validated and approved.
Furthermore, the regulatory landscape is evolving to address emerging concerns related to surfactant use. This includes potential environmental impacts and the development of more sustainable alternatives. Regulatory agencies are increasingly requiring environmental risk assessments for surfactants used in pharmaceutical products, particularly those that may persist in the environment or have potential ecological effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!