How Does Microinjection Molding Improve Pharmaceutical Delivery?
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microinjection Molding in Pharmaceutical Delivery: Background & Objectives
Microinjection molding technology has evolved significantly over the past three decades, emerging from conventional injection molding techniques to address the growing demand for miniaturized components in various industries. In the pharmaceutical sector specifically, this technology has transformed from experimental applications in the early 1990s to becoming a cornerstone of advanced drug delivery systems by the 2020s. The evolution has been driven by the increasing need for precise, patient-friendly, and cost-effective drug delivery mechanisms that can improve therapeutic outcomes while reducing side effects.
The fundamental principle behind microinjection molding involves the precise injection of polymer materials into micro-scale molds to create components with extremely fine features, often measuring less than 1mm and with tolerances in the micrometer range. This level of precision has opened new possibilities for pharmaceutical delivery systems that were previously unattainable with conventional manufacturing methods.
Current technological trends in this field include the integration of smart materials that respond to specific biological triggers, multi-material molding capabilities that combine different polymers in a single component, and the development of biodegradable formulations that dissolve at predetermined rates within the body. These advancements align with the broader pharmaceutical industry's shift toward personalized medicine and targeted therapies.
The primary technical objectives of microinjection molding in pharmaceutical delivery include enhancing drug bioavailability through precise control of release kinetics, improving patient compliance through less invasive and more comfortable delivery systems, and reducing manufacturing costs through process optimization and material efficiency. Additionally, there is a growing focus on developing environmentally sustainable solutions that minimize waste and utilize renewable resources.
From a global perspective, microinjection molding technology has seen varied adoption rates across different regions, with significant research clusters in Western Europe, North America, and East Asia. The technology's development has been characterized by cross-disciplinary collaboration between polymer scientists, pharmaceutical researchers, and manufacturing engineers, leading to innovative approaches that combine insights from multiple fields.
Looking forward, the technology trajectory suggests continued miniaturization, increased functionality through embedded electronics and sensors, and greater customization capabilities to address individual patient needs. These developments are expected to further revolutionize pharmaceutical delivery by enabling more targeted, efficient, and personalized treatment modalities that could significantly improve therapeutic outcomes across a wide range of medical conditions.
The fundamental principle behind microinjection molding involves the precise injection of polymer materials into micro-scale molds to create components with extremely fine features, often measuring less than 1mm and with tolerances in the micrometer range. This level of precision has opened new possibilities for pharmaceutical delivery systems that were previously unattainable with conventional manufacturing methods.
Current technological trends in this field include the integration of smart materials that respond to specific biological triggers, multi-material molding capabilities that combine different polymers in a single component, and the development of biodegradable formulations that dissolve at predetermined rates within the body. These advancements align with the broader pharmaceutical industry's shift toward personalized medicine and targeted therapies.
The primary technical objectives of microinjection molding in pharmaceutical delivery include enhancing drug bioavailability through precise control of release kinetics, improving patient compliance through less invasive and more comfortable delivery systems, and reducing manufacturing costs through process optimization and material efficiency. Additionally, there is a growing focus on developing environmentally sustainable solutions that minimize waste and utilize renewable resources.
From a global perspective, microinjection molding technology has seen varied adoption rates across different regions, with significant research clusters in Western Europe, North America, and East Asia. The technology's development has been characterized by cross-disciplinary collaboration between polymer scientists, pharmaceutical researchers, and manufacturing engineers, leading to innovative approaches that combine insights from multiple fields.
Looking forward, the technology trajectory suggests continued miniaturization, increased functionality through embedded electronics and sensors, and greater customization capabilities to address individual patient needs. These developments are expected to further revolutionize pharmaceutical delivery by enabling more targeted, efficient, and personalized treatment modalities that could significantly improve therapeutic outcomes across a wide range of medical conditions.
Market Demand Analysis for Advanced Drug Delivery Systems
The global market for advanced drug delivery systems is experiencing significant growth, driven by the increasing prevalence of chronic diseases and the need for more effective therapeutic approaches. The current market value for advanced drug delivery technologies exceeds $180 billion and is projected to grow at a compound annual growth rate of 7.8% through 2028. Within this broader market, microinjection molding technology represents a rapidly expanding segment due to its ability to create precise, microscale drug delivery components.
Patient-centric healthcare trends are creating substantial demand for minimally invasive drug delivery methods. Microinjection molding addresses this need by enabling the production of microneedles, implantable devices, and other miniaturized delivery systems that reduce patient discomfort while improving medication adherence. Market research indicates that over 60% of patients prefer less invasive delivery methods, creating a strong commercial incentive for pharmaceutical companies to invest in microinjection molding capabilities.
The biologics sector presents particularly compelling opportunities for microinjection molding applications. With biologics representing approximately 25% of new FDA approvals and commanding premium pricing, pharmaceutical companies are actively seeking delivery technologies capable of preserving these sensitive compounds. Microinjection molding enables the creation of specialized delivery systems that protect biological molecules from degradation while ensuring precise dosing.
Healthcare cost containment pressures are simultaneously driving demand for drug delivery systems that improve therapeutic outcomes while reducing overall treatment costs. Microinjection molded devices that enable targeted drug delivery can significantly reduce the total amount of active pharmaceutical ingredient required, potentially lowering medication costs by 15-30% for certain treatments. This economic benefit is particularly relevant for specialty medications with high production costs.
Regional market analysis reveals varying adoption rates for advanced drug delivery technologies. North America currently leads with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.3% annually, driven by expanding healthcare infrastructure and increasing access to advanced medications.
Regulatory trends are also shaping market demand, with authorities increasingly requiring pharmaceutical companies to demonstrate improved safety profiles and patient outcomes. Microinjection molding supports these requirements by enabling the development of delivery systems with enhanced precision and reliability, potentially accelerating regulatory approval timelines and improving market access for new therapeutic products.
Patient-centric healthcare trends are creating substantial demand for minimally invasive drug delivery methods. Microinjection molding addresses this need by enabling the production of microneedles, implantable devices, and other miniaturized delivery systems that reduce patient discomfort while improving medication adherence. Market research indicates that over 60% of patients prefer less invasive delivery methods, creating a strong commercial incentive for pharmaceutical companies to invest in microinjection molding capabilities.
The biologics sector presents particularly compelling opportunities for microinjection molding applications. With biologics representing approximately 25% of new FDA approvals and commanding premium pricing, pharmaceutical companies are actively seeking delivery technologies capable of preserving these sensitive compounds. Microinjection molding enables the creation of specialized delivery systems that protect biological molecules from degradation while ensuring precise dosing.
Healthcare cost containment pressures are simultaneously driving demand for drug delivery systems that improve therapeutic outcomes while reducing overall treatment costs. Microinjection molded devices that enable targeted drug delivery can significantly reduce the total amount of active pharmaceutical ingredient required, potentially lowering medication costs by 15-30% for certain treatments. This economic benefit is particularly relevant for specialty medications with high production costs.
Regional market analysis reveals varying adoption rates for advanced drug delivery technologies. North America currently leads with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 9.3% annually, driven by expanding healthcare infrastructure and increasing access to advanced medications.
Regulatory trends are also shaping market demand, with authorities increasingly requiring pharmaceutical companies to demonstrate improved safety profiles and patient outcomes. Microinjection molding supports these requirements by enabling the development of delivery systems with enhanced precision and reliability, potentially accelerating regulatory approval timelines and improving market access for new therapeutic products.
Technical Challenges in Microinjection Molding for Pharmaceuticals
Despite significant advancements in microinjection molding for pharmaceutical delivery systems, several technical challenges continue to impede broader implementation and optimization of this technology. The primary challenge lies in achieving consistent micro-feature replication at the submicron level. When molding components with dimensions in the 1-100 micrometer range, conventional molding parameters become inadequate, requiring precise control of melt temperature, injection speed, and holding pressure to ensure complete cavity filling before premature solidification occurs.
Material selection presents another significant hurdle. Pharmaceutical applications demand biocompatible, FDA-approved polymers that must simultaneously possess suitable flow properties for micro-molding. Many medical-grade polymers exhibit high viscosity or narrow processing windows, making them difficult to process at the micro scale without degradation or incomplete filling.
Tool design and fabrication represent particularly demanding challenges. Creating mold inserts with micro-features requires specialized micro-machining techniques such as laser ablation, LIGA processes, or micro-EDM. These tools must maintain extremely tight tolerances, often within ±1-2 micrometers, while also incorporating proper venting and ejection mechanisms that won't damage delicate micro-features during part removal.
Process monitoring and quality control systems face limitations when adapted to microinjection molding. Conventional sensors often lack the sensitivity to detect minor variations that can significantly impact micro-feature quality. In-mold pressure and temperature sensors must be miniaturized without sacrificing accuracy, while vision systems require enhanced resolution to inspect microscopic features effectively.
Surface properties at the micro-scale become increasingly critical for pharmaceutical delivery applications. Controlling surface energy, roughness, and wettability directly impacts drug release kinetics and bioavailability. However, conventional surface treatment methods may not translate effectively to micro-molded components due to scale-dependent physical phenomena.
Production scaling presents unique challenges as well. While laboratory-scale production may achieve acceptable quality, transferring these processes to high-volume manufacturing environments introduces variability that can compromise critical micro-features. Multi-cavity molds must maintain exceptional consistency across all cavities despite the increased complexity.
Post-processing operations such as sterilization can potentially alter the carefully engineered micro-features. Gamma radiation, ethylene oxide, or autoclave sterilization methods may induce polymer chain scission, cross-linking, or dimensional changes that affect drug delivery performance, necessitating specialized validation protocols for these micro-scale components.
Material selection presents another significant hurdle. Pharmaceutical applications demand biocompatible, FDA-approved polymers that must simultaneously possess suitable flow properties for micro-molding. Many medical-grade polymers exhibit high viscosity or narrow processing windows, making them difficult to process at the micro scale without degradation or incomplete filling.
Tool design and fabrication represent particularly demanding challenges. Creating mold inserts with micro-features requires specialized micro-machining techniques such as laser ablation, LIGA processes, or micro-EDM. These tools must maintain extremely tight tolerances, often within ±1-2 micrometers, while also incorporating proper venting and ejection mechanisms that won't damage delicate micro-features during part removal.
Process monitoring and quality control systems face limitations when adapted to microinjection molding. Conventional sensors often lack the sensitivity to detect minor variations that can significantly impact micro-feature quality. In-mold pressure and temperature sensors must be miniaturized without sacrificing accuracy, while vision systems require enhanced resolution to inspect microscopic features effectively.
Surface properties at the micro-scale become increasingly critical for pharmaceutical delivery applications. Controlling surface energy, roughness, and wettability directly impacts drug release kinetics and bioavailability. However, conventional surface treatment methods may not translate effectively to micro-molded components due to scale-dependent physical phenomena.
Production scaling presents unique challenges as well. While laboratory-scale production may achieve acceptable quality, transferring these processes to high-volume manufacturing environments introduces variability that can compromise critical micro-features. Multi-cavity molds must maintain exceptional consistency across all cavities despite the increased complexity.
Post-processing operations such as sterilization can potentially alter the carefully engineered micro-features. Gamma radiation, ethylene oxide, or autoclave sterilization methods may induce polymer chain scission, cross-linking, or dimensional changes that affect drug delivery performance, necessitating specialized validation protocols for these micro-scale components.
Current Microinjection Molding Solutions for Drug Delivery
01 Microinjection molding techniques for pharmaceutical delivery systems
Microinjection molding is used to create precise, miniaturized components for pharmaceutical delivery systems. This manufacturing process allows for the production of complex geometries with high precision and reproducibility, essential for controlled drug release applications. The technique enables the creation of micro-scale features that can enhance drug delivery efficiency while maintaining consistent quality across production batches.- Microinjection molding techniques for pharmaceutical delivery systems: Microinjection molding is used to create precise, miniaturized components for pharmaceutical delivery systems. This manufacturing process allows for the production of complex geometries with high precision and reproducibility, which is essential for controlled drug release. The technique enables the creation of micro-scale devices with features that can enhance drug delivery efficiency and targeting capabilities.
- Biodegradable polymers in microinjection molded drug delivery devices: Biodegradable polymers are utilized in microinjection molding to create pharmaceutical delivery systems that can dissolve or degrade in the body after delivering their payload. These materials provide advantages such as controlled release profiles, biocompatibility, and elimination of the need for device removal. The microinjection molding process allows for precise control of the polymer properties, affecting drug release kinetics and therapeutic efficacy.
- Microneedle arrays for transdermal drug delivery: Microinjection molding is employed to manufacture microneedle arrays for transdermal drug delivery. These arrays consist of numerous tiny needles that can penetrate the skin's outer layer without reaching pain receptors, enabling painless drug administration. The microinjection molding process allows for the creation of microneedles with specific geometries, materials, and drug-loading capabilities, enhancing the efficiency of transdermal delivery systems.
- Microfluidic devices for controlled drug release: Microinjection molding facilitates the production of microfluidic devices with intricate channel networks for controlled pharmaceutical delivery. These devices can precisely regulate drug flow rates, mixing, and release patterns. The high precision of microinjection molding enables the creation of complex microfluidic architectures with features such as valves, pumps, and reservoirs that can respond to specific physiological conditions or external stimuli for smart drug delivery.
- Implantable micro-devices for sustained drug release: Microinjection molding technology is used to create implantable micro-devices for long-term pharmaceutical delivery. These devices can be designed with specific release profiles to maintain therapeutic drug concentrations over extended periods. The precision of microinjection molding allows for the incorporation of features such as drug reservoirs, diffusion barriers, and release-controlling mechanisms that can significantly improve treatment efficacy while reducing side effects and dosing frequency.
02 Microneedle arrays for transdermal drug delivery
Microneedle arrays manufactured through microinjection molding provide minimally invasive transdermal drug delivery solutions. These arrays consist of numerous microscopic needles that can penetrate the skin's outer layer without reaching pain receptors, allowing for painless drug administration. The microinjection molding process enables precise control over needle dimensions, sharpness, and material properties, resulting in effective delivery systems for various pharmaceutical compounds.Expand Specific Solutions03 Biodegradable polymers for controlled release applications
Microinjection molding of biodegradable polymers creates pharmaceutical delivery systems with controlled release properties. These systems can be designed to release drugs at specific rates over predetermined periods, enhancing therapeutic efficacy while reducing side effects. The process allows for the incorporation of active pharmaceutical ingredients directly into the polymer matrix during manufacturing, creating monolithic drug delivery devices with tailored release profiles.Expand Specific Solutions04 Microfluidic devices for precise drug administration
Microinjection molding enables the production of microfluidic devices for precise pharmaceutical delivery. These devices incorporate channels, reservoirs, and valves at the microscale to control fluid flow and drug dispensing with high accuracy. The technology allows for integration of multiple functional components into a single device, facilitating complex drug delivery regimens and responsive dosing based on physiological parameters.Expand Specific Solutions05 Implantable drug delivery systems
Microinjection molding facilitates the production of implantable pharmaceutical delivery systems with complex internal structures. These systems can be designed to provide localized drug delivery directly to target tissues over extended periods, improving therapeutic outcomes while minimizing systemic exposure. The manufacturing process allows for the creation of biocompatible devices with features that promote tissue integration and prevent adverse immune responses.Expand Specific Solutions
Key Industry Players in Pharmaceutical Microinjection Molding
Microinjection molding technology in pharmaceutical delivery is evolving rapidly, currently transitioning from early adoption to growth phase. The global market is expanding significantly, projected to reach several billion dollars by 2025, driven by demand for precise drug delivery systems. Technologically, the field shows varying maturity levels across players. Industry leaders like Becton, Dickinson & Co. and F. Hoffmann-La Roche demonstrate advanced capabilities in commercial applications, while research institutions such as MIT, Johns Hopkins University, and Chinese Academy of Sciences are pioneering next-generation innovations. Emerging companies like Feroka and Nextbiomedical are developing specialized applications including microneedle patches. The competitive landscape features both established pharmaceutical giants and innovative startups, with significant research collaboration between industry and academia driving technological advancement.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. (BD) has developed advanced microinjection molding technologies for pharmaceutical delivery systems that enable the production of microneedle arrays with precise geometries at the microscale. Their proprietary microinjection molding process allows for the creation of biodegradable polymer microneedles with controlled dimensions (typically 500-900 μm in length) and sharp tip radii (less than 10 μm). BD's technology incorporates specialized mold designs with micro-cavities and vacuum-assisted filling to ensure complete mold filling and prevent air entrapment. Their systems integrate drug formulations directly into the molding process, enabling the production of drug-loaded microneedles with precise dosing capabilities. BD has also developed multi-material microinjection molding techniques that create microneedles with layered structures for controlled drug release profiles, allowing for sequential delivery of multiple therapeutic agents from a single device.
Strengths: Superior precision in microneedle dimensions and geometry control; scalable manufacturing process suitable for mass production; excellent reproducibility between production batches. Weaknesses: Higher initial tooling costs compared to other microfabrication methods; limited to thermoplastic materials with suitable flow properties; potential drug degradation during processing due to heat exposure.
F. Hoffmann-La Roche Ltd.
Technical Solution: F. Hoffmann-La Roche has pioneered microinjection molding technology for pharmaceutical delivery through their development of polymer-based microstructured delivery systems. Their approach utilizes specialized high-precision microinjection molding equipment capable of producing features down to 5 μm with tight tolerances. Roche's technology incorporates pharmaceutical active ingredients directly into the polymer matrix before the molding process, ensuring homogeneous distribution throughout the microstructures. Their proprietary process employs specialized polymers with optimized rheological properties that maintain drug stability during the high-pressure, high-temperature molding process. Roche has developed multi-cavity molds that enable high-throughput production of microneedle arrays and microreservoirs with consistent quality. Their systems include specialized surface treatments applied post-molding to enhance biocompatibility and control drug release kinetics. Additionally, Roche has integrated sensors within their microinjection molded delivery systems to provide real-time monitoring of drug delivery parameters and patient compliance data.
Strengths: Excellent integration of active pharmaceutical ingredients with polymer matrices; highly scalable manufacturing process suitable for commercial production; sophisticated quality control systems ensuring batch consistency. Weaknesses: Complex process validation requirements; higher production costs compared to conventional delivery systems; limited to drugs that can withstand processing conditions.
Critical Patents and Innovations in Pharmaceutical Microinjection
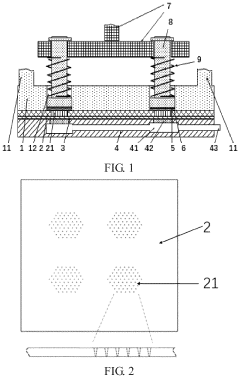
Strongly hydrophilic microneedle substrate and drug-loaded microneedle and use thereof in treatment of diseases
PatentPendingUS20240245754A1
Innovation
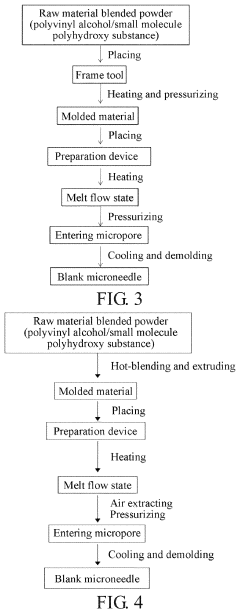
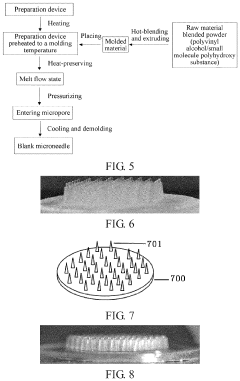
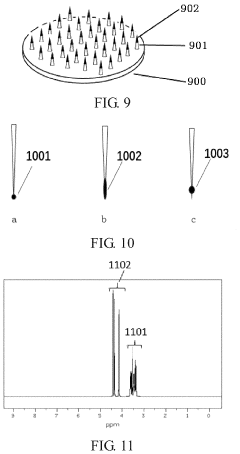
- A strongly hydrophilic microneedle substrate composition made from polyvinyl alcohol and a small molecule polyhydroxy compound, with a hydroxyl ratio of 0.1:1 to 1.3:1, is used to create a blank microneedle that can be easily loaded with drugs, enhancing adhesion and drug retention without high-temperature processing.
Micro-electroporation based drug delivery system, methods for use and fabrication thereof
PatentPendingUS20230381476A1
Innovation
- An electrically conductive microneedle platform that serves as both electrodes and microprotrusions for in situ electroporation, using a hydrogel-based conductive polymer composition to deliver nucleic acid vaccines without the need for carrier molecules, allowing for controlled release through electro-osmosis under low-voltage stimulation.
Regulatory Compliance and Approval Pathways
Microinjection molding technologies for pharmaceutical delivery systems must navigate a complex regulatory landscape to reach market approval. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established specific guidelines for combination products that integrate drug delivery devices with pharmaceutical compounds. These regulatory frameworks evaluate both the device components and the drug formulations, requiring manufacturers to demonstrate safety, efficacy, and quality through comprehensive testing protocols.
For microinjection molded components in pharmaceutical delivery systems, manufacturers must comply with ISO 13485 standards for medical device quality management systems and ISO 10993 for biocompatibility testing. The choice of polymer materials is particularly scrutinized, with regulatory bodies requiring extensive documentation on leachables and extractables that might interact with drug formulations or patient tissues.
The regulatory pathway typically depends on the classification of the delivery system. Novel microinjection molded delivery systems often follow the 510(k) pathway in the U.S. if substantially equivalent to predicate devices, or the more rigorous Premarket Approval (PMA) process for innovative technologies. In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval process, with classification determining the level of scrutiny.
Manufacturing process validation represents another critical regulatory consideration. Microinjection molding processes must demonstrate consistent production capabilities with minimal variability, as regulatory agencies require validation of critical process parameters that might affect product quality or performance. This includes validation of sterilization methods compatible with the microinjection molded components, which must maintain structural integrity and functionality after sterilization.
Accelerated approval pathways exist for breakthrough technologies that address unmet medical needs. The FDA's Breakthrough Devices Program and the EMA's PRIME (PRIority MEdicines) scheme can expedite regulatory review for innovative microinjection molding technologies that significantly improve pharmaceutical delivery for serious conditions. These pathways offer increased regulatory interaction and guidance throughout the development process.
Post-market surveillance requirements have become increasingly stringent, with manufacturers needing to implement robust systems for monitoring device performance and adverse events. For microinjection molded pharmaceutical delivery systems, this includes tracking potential issues related to material degradation, mechanical failures, or unexpected drug-device interactions that might emerge only after extended clinical use.
International harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), are gradually streamlining global regulatory processes, potentially reducing the burden for manufacturers seeking multi-market approvals for microinjection molded pharmaceutical delivery technologies.
For microinjection molded components in pharmaceutical delivery systems, manufacturers must comply with ISO 13485 standards for medical device quality management systems and ISO 10993 for biocompatibility testing. The choice of polymer materials is particularly scrutinized, with regulatory bodies requiring extensive documentation on leachables and extractables that might interact with drug formulations or patient tissues.
The regulatory pathway typically depends on the classification of the delivery system. Novel microinjection molded delivery systems often follow the 510(k) pathway in the U.S. if substantially equivalent to predicate devices, or the more rigorous Premarket Approval (PMA) process for innovative technologies. In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern the approval process, with classification determining the level of scrutiny.
Manufacturing process validation represents another critical regulatory consideration. Microinjection molding processes must demonstrate consistent production capabilities with minimal variability, as regulatory agencies require validation of critical process parameters that might affect product quality or performance. This includes validation of sterilization methods compatible with the microinjection molded components, which must maintain structural integrity and functionality after sterilization.
Accelerated approval pathways exist for breakthrough technologies that address unmet medical needs. The FDA's Breakthrough Devices Program and the EMA's PRIME (PRIority MEdicines) scheme can expedite regulatory review for innovative microinjection molding technologies that significantly improve pharmaceutical delivery for serious conditions. These pathways offer increased regulatory interaction and guidance throughout the development process.
Post-market surveillance requirements have become increasingly stringent, with manufacturers needing to implement robust systems for monitoring device performance and adverse events. For microinjection molded pharmaceutical delivery systems, this includes tracking potential issues related to material degradation, mechanical failures, or unexpected drug-device interactions that might emerge only after extended clinical use.
International harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), are gradually streamlining global regulatory processes, potentially reducing the burden for manufacturers seeking multi-market approvals for microinjection molded pharmaceutical delivery technologies.
Biocompatibility and Material Science Considerations
Biocompatibility represents a critical factor in the development of microinjection molded components for pharmaceutical delivery systems. The materials selected must not only withstand the molding process but also remain inert when in contact with biological tissues and pharmaceutical compounds. Polymers such as medical-grade polycarbonate (PC), polyetheretherketone (PEEK), cyclic olefin copolymer (COC), and liquid crystal polymers (LCP) have emerged as preferred choices due to their excellent biocompatibility profiles and mechanical properties.
Material science advancements have significantly expanded the range of biocompatible materials suitable for microinjection molding. These materials undergo rigorous testing protocols including cytotoxicity, sensitization, irritation, and systemic toxicity evaluations to ensure compliance with ISO 10993 standards. The surface properties of these materials can be further modified through techniques such as plasma treatment or coating applications to enhance drug compatibility or control release kinetics.
The interface between material science and pharmaceutical efficacy presents unique challenges in microinjection molding. Researchers must consider potential leachables and extractables that might compromise drug stability or patient safety. Advanced analytical techniques including high-performance liquid chromatography (HPLC) and mass spectrometry are employed to detect trace contaminants that could potentially migrate from the polymer matrix into the pharmaceutical formulation.
Degradation characteristics represent another crucial consideration, particularly for biodegradable delivery systems. Materials such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers offer controlled degradation profiles that can be tailored to match desired drug release rates. The microinjection molding process parameters must be precisely controlled to maintain these degradation properties while achieving the required microstructural features.
Thermal stability during processing presents a significant material science challenge. The high temperatures and shear forces involved in microinjection molding can potentially degrade both the polymer and any incorporated pharmaceutical agents. This necessitates careful selection of processing windows that preserve material integrity while achieving the required flow characteristics for micromolding complex geometries.
Recent innovations in composite materials have expanded the functional capabilities of microinjection molded drug delivery systems. Polymer-ceramic composites, for instance, combine the processability of polymers with enhanced mechanical properties and bioactivity. These advanced materials enable the creation of multifunctional delivery systems that can simultaneously provide structural support, controlled release, and even therapeutic effects through the incorporation of bioactive components.
Material science advancements have significantly expanded the range of biocompatible materials suitable for microinjection molding. These materials undergo rigorous testing protocols including cytotoxicity, sensitization, irritation, and systemic toxicity evaluations to ensure compliance with ISO 10993 standards. The surface properties of these materials can be further modified through techniques such as plasma treatment or coating applications to enhance drug compatibility or control release kinetics.
The interface between material science and pharmaceutical efficacy presents unique challenges in microinjection molding. Researchers must consider potential leachables and extractables that might compromise drug stability or patient safety. Advanced analytical techniques including high-performance liquid chromatography (HPLC) and mass spectrometry are employed to detect trace contaminants that could potentially migrate from the polymer matrix into the pharmaceutical formulation.
Degradation characteristics represent another crucial consideration, particularly for biodegradable delivery systems. Materials such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers offer controlled degradation profiles that can be tailored to match desired drug release rates. The microinjection molding process parameters must be precisely controlled to maintain these degradation properties while achieving the required microstructural features.
Thermal stability during processing presents a significant material science challenge. The high temperatures and shear forces involved in microinjection molding can potentially degrade both the polymer and any incorporated pharmaceutical agents. This necessitates careful selection of processing windows that preserve material integrity while achieving the required flow characteristics for micromolding complex geometries.
Recent innovations in composite materials have expanded the functional capabilities of microinjection molded drug delivery systems. Polymer-ceramic composites, for instance, combine the processability of polymers with enhanced mechanical properties and bioactivity. These advanced materials enable the creation of multifunctional delivery systems that can simultaneously provide structural support, controlled release, and even therapeutic effects through the incorporation of bioactive components.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!