How Hydroxyapatite Improves Toughness in Biodegradable Bone Grafts
SEP 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite in Bone Grafts: Background and Objectives
Hydroxyapatite (HA) has emerged as a crucial component in the development of biodegradable bone grafts, revolutionizing the field of orthopedic and dental implants. This naturally occurring mineral, primarily composed of calcium and phosphate, closely resembles the inorganic constituent of human bone tissue. The integration of HA into biodegradable bone grafts aims to enhance their mechanical properties, particularly toughness, while maintaining biocompatibility and osteoconductivity.
The evolution of bone graft materials has been driven by the need to address limitations in traditional autografts and allografts, such as limited availability, donor site morbidity, and potential disease transmission. Synthetic bone graft substitutes, including biodegradable polymers and ceramics, have gained significant attention in recent decades. However, these materials often lack the necessary mechanical strength and biological properties to fully mimic natural bone tissue.
Hydroxyapatite's role in improving the toughness of biodegradable bone grafts stems from its unique structural and chemical properties. When incorporated into biodegradable polymers or other matrix materials, HA particles can act as reinforcing agents, enhancing the overall mechanical strength and fracture resistance of the composite. This synergistic combination aims to create bone graft materials that not only provide temporary structural support but also promote bone regeneration and integration.
The primary objective of incorporating HA into biodegradable bone grafts is to develop materials that closely mimic the natural bone environment. This includes achieving a balance between mechanical properties, biodegradability, and bioactivity. By improving toughness, HA-enhanced grafts can better withstand physiological loads and maintain their structural integrity during the bone healing process. Additionally, the presence of HA can promote osteoblast adhesion, proliferation, and differentiation, facilitating faster and more effective bone regeneration.
Research in this field has been driven by the increasing demand for advanced biomaterials in orthopedic and dental applications. The aging population and rising incidence of bone-related disorders have further accelerated the need for innovative bone graft solutions. As such, understanding the mechanisms by which HA improves toughness in biodegradable bone grafts is crucial for developing next-generation implant materials that can effectively support bone healing and regeneration.
The technological trajectory in this domain focuses on optimizing the composition, structure, and processing of HA-containing biodegradable composites. This includes exploring various HA particle sizes, morphologies, and distributions within the matrix material to maximize toughness enhancement. Furthermore, researchers are investigating the synergistic effects of combining HA with other bioactive components, such as growth factors or stem cells, to create multifunctional bone graft materials with superior mechanical and biological properties.
The evolution of bone graft materials has been driven by the need to address limitations in traditional autografts and allografts, such as limited availability, donor site morbidity, and potential disease transmission. Synthetic bone graft substitutes, including biodegradable polymers and ceramics, have gained significant attention in recent decades. However, these materials often lack the necessary mechanical strength and biological properties to fully mimic natural bone tissue.
Hydroxyapatite's role in improving the toughness of biodegradable bone grafts stems from its unique structural and chemical properties. When incorporated into biodegradable polymers or other matrix materials, HA particles can act as reinforcing agents, enhancing the overall mechanical strength and fracture resistance of the composite. This synergistic combination aims to create bone graft materials that not only provide temporary structural support but also promote bone regeneration and integration.
The primary objective of incorporating HA into biodegradable bone grafts is to develop materials that closely mimic the natural bone environment. This includes achieving a balance between mechanical properties, biodegradability, and bioactivity. By improving toughness, HA-enhanced grafts can better withstand physiological loads and maintain their structural integrity during the bone healing process. Additionally, the presence of HA can promote osteoblast adhesion, proliferation, and differentiation, facilitating faster and more effective bone regeneration.
Research in this field has been driven by the increasing demand for advanced biomaterials in orthopedic and dental applications. The aging population and rising incidence of bone-related disorders have further accelerated the need for innovative bone graft solutions. As such, understanding the mechanisms by which HA improves toughness in biodegradable bone grafts is crucial for developing next-generation implant materials that can effectively support bone healing and regeneration.
The technological trajectory in this domain focuses on optimizing the composition, structure, and processing of HA-containing biodegradable composites. This includes exploring various HA particle sizes, morphologies, and distributions within the matrix material to maximize toughness enhancement. Furthermore, researchers are investigating the synergistic effects of combining HA with other bioactive components, such as growth factors or stem cells, to create multifunctional bone graft materials with superior mechanical and biological properties.
Market Analysis for Advanced Biodegradable Bone Grafts
The market for advanced biodegradable bone grafts incorporating hydroxyapatite has shown significant growth potential in recent years. This trend is driven by the increasing prevalence of bone-related disorders, rising geriatric population, and growing demand for minimally invasive surgical procedures. The global bone graft substitutes market, which includes biodegradable options, was valued at approximately $2.7 billion in 2020 and is projected to reach $3.9 billion by 2026, with a compound annual growth rate (CAGR) of around 6.3%.
Hydroxyapatite-enhanced biodegradable bone grafts are gaining traction due to their superior biocompatibility, osteoconductivity, and improved mechanical properties. These advanced grafts address the limitations of traditional autografts and allografts, such as donor site morbidity and limited availability. The market for these innovative products is particularly strong in North America and Europe, where advanced healthcare infrastructure and higher healthcare expenditure support the adoption of cutting-edge medical technologies.
The orthopedic segment dominates the biodegradable bone graft market, accounting for the largest share due to the high incidence of orthopedic injuries and disorders. Dental applications are also showing rapid growth, driven by the increasing demand for dental implants and reconstructive surgeries. Spinal fusion procedures represent another significant market segment, with a growing number of patients seeking treatment for degenerative disc diseases and other spinal disorders.
Key market players in this space include Medtronic, Stryker, DePuy Synthes (Johnson & Johnson), Zimmer Biomet, and Geistlich Pharma AG. These companies are investing heavily in research and development to enhance the performance of biodegradable bone grafts through the incorporation of hydroxyapatite and other bioactive materials. Smaller, specialized companies are also making significant contributions to innovation in this field, often partnering with larger firms for commercialization and distribution.
The market is witnessing a shift towards personalized medicine, with an increasing focus on patient-specific bone graft solutions. This trend is supported by advancements in 3D printing technologies, which allow for the fabrication of custom-designed grafts that closely match the patient's anatomy and specific requirements. The integration of hydroxyapatite in these personalized grafts is expected to further enhance their performance and market appeal.
Regulatory approval processes and reimbursement policies play a crucial role in market dynamics. While stringent regulations ensure product safety and efficacy, they can also pose challenges for market entry and product development timelines. However, favorable reimbursement policies in developed countries are expected to drive market growth by improving patient access to these advanced bone graft solutions.
Hydroxyapatite-enhanced biodegradable bone grafts are gaining traction due to their superior biocompatibility, osteoconductivity, and improved mechanical properties. These advanced grafts address the limitations of traditional autografts and allografts, such as donor site morbidity and limited availability. The market for these innovative products is particularly strong in North America and Europe, where advanced healthcare infrastructure and higher healthcare expenditure support the adoption of cutting-edge medical technologies.
The orthopedic segment dominates the biodegradable bone graft market, accounting for the largest share due to the high incidence of orthopedic injuries and disorders. Dental applications are also showing rapid growth, driven by the increasing demand for dental implants and reconstructive surgeries. Spinal fusion procedures represent another significant market segment, with a growing number of patients seeking treatment for degenerative disc diseases and other spinal disorders.
Key market players in this space include Medtronic, Stryker, DePuy Synthes (Johnson & Johnson), Zimmer Biomet, and Geistlich Pharma AG. These companies are investing heavily in research and development to enhance the performance of biodegradable bone grafts through the incorporation of hydroxyapatite and other bioactive materials. Smaller, specialized companies are also making significant contributions to innovation in this field, often partnering with larger firms for commercialization and distribution.
The market is witnessing a shift towards personalized medicine, with an increasing focus on patient-specific bone graft solutions. This trend is supported by advancements in 3D printing technologies, which allow for the fabrication of custom-designed grafts that closely match the patient's anatomy and specific requirements. The integration of hydroxyapatite in these personalized grafts is expected to further enhance their performance and market appeal.
Regulatory approval processes and reimbursement policies play a crucial role in market dynamics. While stringent regulations ensure product safety and efficacy, they can also pose challenges for market entry and product development timelines. However, favorable reimbursement policies in developed countries are expected to drive market growth by improving patient access to these advanced bone graft solutions.
Current Challenges in Biodegradable Bone Graft Toughness
Biodegradable bone grafts have shown great promise in orthopedic applications, offering a temporary scaffold that supports bone regeneration while gradually degrading over time. However, a significant challenge in their development has been achieving sufficient toughness to withstand physiological loads without compromising their biodegradability. This issue has been a major focus of research in the field of biomaterials and tissue engineering.
One of the primary challenges is the inherent brittleness of many biodegradable polymers used in bone graft materials. Materials such as polylactic acid (PLA) and polyglycolic acid (PGA) often lack the necessary toughness to withstand the complex mechanical stresses experienced in load-bearing bone applications. This brittleness can lead to premature failure of the graft, potentially compromising the healing process and patient outcomes.
Another significant challenge is maintaining an appropriate balance between mechanical properties and degradation rate. Enhancing the toughness of biodegradable bone grafts often involves modifications that can alter their degradation profile. Achieving a material that is both tough enough to support physiological loads and degrades at a rate that matches new bone formation remains a complex engineering problem.
The incorporation of bioactive ceramics, such as hydroxyapatite, into biodegradable polymers has been explored as a potential solution. While this approach can improve the overall mechanical properties and bioactivity of the graft, it also introduces new challenges. These include ensuring uniform distribution of the ceramic phase within the polymer matrix and preventing agglomeration, which can create stress concentration points and potentially weaken the overall structure.
Furthermore, the interface between the polymer matrix and ceramic particles presents its own set of challenges. Poor adhesion between these phases can lead to premature failure and reduced toughness. Developing methods to enhance this interfacial bonding without compromising the biodegradability or biocompatibility of the graft material is an ongoing area of research.
The dynamic nature of the in vivo environment adds another layer of complexity to the toughness challenge. As the graft material begins to degrade, its mechanical properties change over time. Designing a material that maintains sufficient toughness throughout the degradation process, while still allowing for gradual replacement by native bone tissue, requires a deep understanding of both material science and bone biology.
Lastly, the scalability and reproducibility of toughened biodegradable bone grafts present significant challenges for clinical translation. Developing manufacturing processes that can consistently produce grafts with the desired mechanical properties, while meeting regulatory standards for medical devices, remains a hurdle in bringing these advanced materials from the laboratory to the clinic.
One of the primary challenges is the inherent brittleness of many biodegradable polymers used in bone graft materials. Materials such as polylactic acid (PLA) and polyglycolic acid (PGA) often lack the necessary toughness to withstand the complex mechanical stresses experienced in load-bearing bone applications. This brittleness can lead to premature failure of the graft, potentially compromising the healing process and patient outcomes.
Another significant challenge is maintaining an appropriate balance between mechanical properties and degradation rate. Enhancing the toughness of biodegradable bone grafts often involves modifications that can alter their degradation profile. Achieving a material that is both tough enough to support physiological loads and degrades at a rate that matches new bone formation remains a complex engineering problem.
The incorporation of bioactive ceramics, such as hydroxyapatite, into biodegradable polymers has been explored as a potential solution. While this approach can improve the overall mechanical properties and bioactivity of the graft, it also introduces new challenges. These include ensuring uniform distribution of the ceramic phase within the polymer matrix and preventing agglomeration, which can create stress concentration points and potentially weaken the overall structure.
Furthermore, the interface between the polymer matrix and ceramic particles presents its own set of challenges. Poor adhesion between these phases can lead to premature failure and reduced toughness. Developing methods to enhance this interfacial bonding without compromising the biodegradability or biocompatibility of the graft material is an ongoing area of research.
The dynamic nature of the in vivo environment adds another layer of complexity to the toughness challenge. As the graft material begins to degrade, its mechanical properties change over time. Designing a material that maintains sufficient toughness throughout the degradation process, while still allowing for gradual replacement by native bone tissue, requires a deep understanding of both material science and bone biology.
Lastly, the scalability and reproducibility of toughened biodegradable bone grafts present significant challenges for clinical translation. Developing manufacturing processes that can consistently produce grafts with the desired mechanical properties, while meeting regulatory standards for medical devices, remains a hurdle in bringing these advanced materials from the laboratory to the clinic.
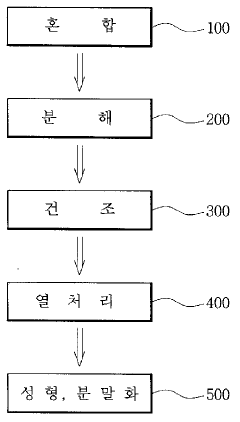
Existing Hydroxyapatite Incorporation Techniques
01 Composite materials for improved hydroxyapatite toughness
Incorporating various materials into hydroxyapatite to create composites can significantly enhance its toughness. These composites often combine the biocompatibility of hydroxyapatite with the mechanical strength of other materials, resulting in improved overall toughness. Common additives include polymers, metals, and other ceramics, which can create a more resilient and durable material suitable for various biomedical applications.- Composite materials for improved hydroxyapatite toughness: Incorporating various materials into hydroxyapatite to create composites can significantly enhance its toughness. These composites often combine the biocompatibility of hydroxyapatite with the mechanical strength of other materials, resulting in improved overall toughness. Common additives include polymers, metals, and other ceramics, which can create a more resilient structure.
- Surface modification techniques for hydroxyapatite: Various surface modification techniques can be applied to hydroxyapatite to enhance its toughness. These methods may include coating, ion implantation, or chemical treatments that alter the surface properties of hydroxyapatite. Such modifications can improve the material's resistance to crack propagation and increase its overall toughness.
- Nanostructured hydroxyapatite for increased toughness: Developing hydroxyapatite with nanostructured features can lead to improved toughness. By controlling the grain size and morphology at the nanoscale, it's possible to create hydroxyapatite materials with enhanced mechanical properties, including increased fracture toughness and resistance to crack propagation.
- Sintering processes for toughness enhancement: Optimizing sintering processes can significantly improve the toughness of hydroxyapatite. Advanced sintering techniques, such as spark plasma sintering or microwave sintering, can lead to denser materials with improved mechanical properties. Controlling sintering parameters like temperature, pressure, and time can result in hydroxyapatite with enhanced toughness.
- Biomimetic approaches to hydroxyapatite toughness: Biomimetic strategies inspired by natural bone structures can be employed to enhance hydroxyapatite toughness. These approaches often involve mimicking the hierarchical structure of bone, incorporating organic components, or replicating the mineralization process found in nature. Such biomimetic methods can lead to hydroxyapatite materials with improved toughness and mechanical properties similar to natural bone.
02 Surface modification techniques for hydroxyapatite
Various surface modification techniques can be employed to enhance the toughness of hydroxyapatite. These methods may include coating, ion implantation, or chemical treatments that alter the surface properties of hydroxyapatite. Such modifications can improve the material's resistance to crack propagation, enhance its bonding with other materials, and increase its overall toughness without compromising its biocompatibility.Expand Specific Solutions03 Nanostructured hydroxyapatite for increased toughness
Developing nanostructured forms of hydroxyapatite can lead to significant improvements in toughness. By controlling the size and arrangement of hydroxyapatite crystals at the nanoscale, researchers can create materials with enhanced mechanical properties. These nanostructured materials often exhibit improved fracture resistance and overall toughness compared to conventional hydroxyapatite, making them suitable for load-bearing applications in biomedicine.Expand Specific Solutions04 Heat treatment processes for toughening hydroxyapatite
Various heat treatment processes can be applied to hydroxyapatite to improve its toughness. These thermal treatments may include sintering, annealing, or controlled cooling, which can affect the material's microstructure, density, and grain size. By optimizing these heat treatment parameters, it is possible to enhance the toughness of hydroxyapatite while maintaining its desirable bioactive properties.Expand Specific Solutions05 Doping hydroxyapatite with other elements
Incorporating small amounts of other elements into the hydroxyapatite crystal structure through doping can significantly improve its toughness. Common dopants include various metal ions or rare earth elements. These dopants can alter the material's lattice structure, grain boundaries, and overall mechanical properties, resulting in enhanced toughness while preserving the biocompatibility of hydroxyapatite.Expand Specific Solutions
Key Players in Biodegradable Bone Graft Industry
The development of hydroxyapatite-enhanced biodegradable bone grafts is in a growth phase, with increasing market size and technological advancements. The global bone graft substitutes market, valued at $2.7 billion in 2020, is expected to reach $3.9 billion by 2026. Technological maturity varies among key players, with academic institutions like Zhejiang University and South China University of Technology leading in research, while companies such as Bioventus LLC and Zimmer Orthobiologics, Inc. focus on commercialization. The competitive landscape includes a mix of universities, research institutes, and medical technology companies, indicating a collaborative environment for innovation in this field.
Zhejiang University
Technical Solution: Researchers at Zhejiang University have developed an innovative approach to improve the toughness of biodegradable bone grafts using hydroxyapatite (HA). Their method involves creating a nanocomposite material by combining HA nanoparticles with a biodegradable polymer matrix, such as poly(lactic acid) (PLA) or poly(ε-caprolactone) (PCL). The team has implemented a unique surface modification technique for the HA nanoparticles, which enhances their interfacial bonding with the polymer matrix[8]. This improved bonding leads to better load transfer and increased overall toughness of the bone graft. Additionally, they have developed a novel processing method that allows for the creation of a gradient structure within the graft, mimicking the natural variation in bone density and mechanical properties[9]. This gradient structure further enhances the toughness and promotes better integration with surrounding tissue.
Strengths: Enhanced interfacial bonding, gradient structure mimicking natural bone, improved load transfer. Weaknesses: May require specialized equipment for production, potential challenges in regulatory approval due to novel processing methods.
Chinese Academy of Science Institute of Chemistry
Technical Solution: The Chinese Academy of Science Institute of Chemistry has developed a cutting-edge approach to enhance the toughness of biodegradable bone grafts using hydroxyapatite (HA). Their method involves creating a nanocomposite material that combines HA nanoparticles with a biodegradable polymer matrix and graphene oxide (GO) nanosheets. The addition of GO significantly improves the mechanical properties and toughness of the bone graft material[10]. The researchers have developed a unique in situ synthesis method that allows for the uniform distribution of HA nanoparticles and GO within the polymer matrix, resulting in enhanced load-bearing capabilities and fracture resistance. Furthermore, they have implemented a surface functionalization technique for the HA nanoparticles, which improves their compatibility with the polymer matrix and promotes better cell adhesion and proliferation[11]. This multifaceted approach results in a bone graft material with superior toughness, biocompatibility, and osteogenic properties.
Strengths: Incorporation of graphene oxide for enhanced mechanical properties, uniform distribution of nanoparticles, improved cell adhesion. Weaknesses: Potential concerns about the long-term effects of graphene oxide in the body, may require extensive safety studies.
Innovations in Hydroxyapatite-Enhanced Bone Grafts
Bone graft materials using micro hydroxyapatite and manufacturing method thereof
PatentInactiveKR1020090061333A
Innovation
- Utilization of micro hydroxyapatite for bone graft materials, providing excellent in vivo stability and mechanical properties.
- Cost-effective manufacturing process using abundant calcium sources and hydroxyapatite with excellent sinterability.
- Versatile application of the biomaterial powder in various fields including artificial bone, tooth, water treatment, cosmetics, and medical supplies.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing and implementing hydroxyapatite-enhanced biodegradable bone grafts. The integration of hydroxyapatite into these grafts necessitates a thorough evaluation of potential biological interactions and long-term effects on the host tissue.
One of the primary concerns is the potential for immune responses or inflammatory reactions to the hydroxyapatite component. While hydroxyapatite is generally considered biocompatible due to its similarity to natural bone mineral, the specific formulation and manufacturing process can influence its immunogenicity. Researchers must carefully assess the purity and particle size of the hydroxyapatite used, as these factors can impact the body's response to the material.
The degradation profile of the composite graft is another critical aspect of biocompatibility. As the biodegradable matrix breaks down, the release kinetics of hydroxyapatite particles must be carefully controlled to prevent localized accumulation or systemic distribution. Excessive release of hydroxyapatite could potentially lead to ectopic calcification or other undesired effects in surrounding tissues.
Long-term safety studies are essential to evaluate the potential for adverse effects over extended periods. This includes monitoring for any signs of toxicity, carcinogenicity, or genotoxicity associated with the hydroxyapatite-enhanced grafts. Additionally, the impact on bone remodeling and the potential for abnormal bone formation must be thoroughly investigated to ensure that the improved toughness does not compromise the natural healing process.
The mechanical properties of the composite graft must also be considered from a safety perspective. While hydroxyapatite aims to enhance toughness, it is crucial to ensure that the overall mechanical behavior of the graft remains compatible with the surrounding bone tissue. Mismatches in stiffness or strength could lead to stress shielding or fracture, potentially compromising the healing process and patient safety.
Sterilization methods for hydroxyapatite-enhanced biodegradable bone grafts require careful consideration. The chosen sterilization technique must effectively eliminate pathogens without altering the chemical composition or physical properties of the composite material. This is particularly challenging given the organic nature of the biodegradable matrix and the potential sensitivity of hydroxyapatite to certain sterilization methods.
Regulatory compliance is a critical aspect of ensuring the safety of these advanced bone graft materials. Developers must adhere to stringent guidelines set by regulatory bodies such as the FDA or EMA, which may require extensive preclinical and clinical studies to demonstrate both short-term and long-term safety profiles. This includes comprehensive biocompatibility testing in accordance with ISO 10993 standards and other relevant regulatory frameworks.
One of the primary concerns is the potential for immune responses or inflammatory reactions to the hydroxyapatite component. While hydroxyapatite is generally considered biocompatible due to its similarity to natural bone mineral, the specific formulation and manufacturing process can influence its immunogenicity. Researchers must carefully assess the purity and particle size of the hydroxyapatite used, as these factors can impact the body's response to the material.
The degradation profile of the composite graft is another critical aspect of biocompatibility. As the biodegradable matrix breaks down, the release kinetics of hydroxyapatite particles must be carefully controlled to prevent localized accumulation or systemic distribution. Excessive release of hydroxyapatite could potentially lead to ectopic calcification or other undesired effects in surrounding tissues.
Long-term safety studies are essential to evaluate the potential for adverse effects over extended periods. This includes monitoring for any signs of toxicity, carcinogenicity, or genotoxicity associated with the hydroxyapatite-enhanced grafts. Additionally, the impact on bone remodeling and the potential for abnormal bone formation must be thoroughly investigated to ensure that the improved toughness does not compromise the natural healing process.
The mechanical properties of the composite graft must also be considered from a safety perspective. While hydroxyapatite aims to enhance toughness, it is crucial to ensure that the overall mechanical behavior of the graft remains compatible with the surrounding bone tissue. Mismatches in stiffness or strength could lead to stress shielding or fracture, potentially compromising the healing process and patient safety.
Sterilization methods for hydroxyapatite-enhanced biodegradable bone grafts require careful consideration. The chosen sterilization technique must effectively eliminate pathogens without altering the chemical composition or physical properties of the composite material. This is particularly challenging given the organic nature of the biodegradable matrix and the potential sensitivity of hydroxyapatite to certain sterilization methods.
Regulatory compliance is a critical aspect of ensuring the safety of these advanced bone graft materials. Developers must adhere to stringent guidelines set by regulatory bodies such as the FDA or EMA, which may require extensive preclinical and clinical studies to demonstrate both short-term and long-term safety profiles. This includes comprehensive biocompatibility testing in accordance with ISO 10993 standards and other relevant regulatory frameworks.
Regulatory Pathway for Novel Bone Graft Materials
The regulatory pathway for novel bone graft materials incorporating hydroxyapatite to improve toughness in biodegradable scaffolds involves a complex process of evaluation and approval. This pathway is primarily overseen by regulatory bodies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), depending on the intended market.
Initially, developers must classify their bone graft material according to regulatory guidelines. In the United States, most bone graft substitutes are considered Class II medical devices, requiring a 510(k) premarket notification. However, if the material contains biological components or is significantly different from existing products, it may be classified as a Class III device, necessitating a more rigorous Premarket Approval (PMA) process.
The next step involves conducting extensive preclinical studies to demonstrate the safety and efficacy of the hydroxyapatite-enhanced biodegradable bone graft. These studies typically include in vitro tests to assess biocompatibility, cytotoxicity, and material properties, as well as in vivo animal studies to evaluate osseointegration, biodegradation rates, and potential adverse effects.
Following successful preclinical trials, developers must design and conduct clinical trials. The FDA's Investigational Device Exemption (IDE) process allows for the use of investigational devices in human clinical studies. These trials are crucial for demonstrating the safety and effectiveness of the novel bone graft material in human subjects.
Throughout the development process, manufacturers must adhere to Good Manufacturing Practices (GMP) and establish a robust quality management system. This ensures consistency in production and helps maintain the quality and safety of the final product.
The regulatory submission package must include comprehensive data from preclinical and clinical studies, detailed information on the manufacturing process, and a thorough risk assessment. Regulatory agencies will review this package, often engaging in a dialogue with the developers to address any concerns or request additional information.
Post-market surveillance is a critical component of the regulatory pathway. Manufacturers are required to monitor the performance of their bone graft materials after market approval, reporting any adverse events or unexpected outcomes to the regulatory authorities.
Lastly, it's important to note that regulatory requirements may vary between different countries and regions. Developers aiming for global markets must consider these variations and potentially pursue multiple regulatory approvals simultaneously to ensure widespread availability of their innovative bone graft materials.
Initially, developers must classify their bone graft material according to regulatory guidelines. In the United States, most bone graft substitutes are considered Class II medical devices, requiring a 510(k) premarket notification. However, if the material contains biological components or is significantly different from existing products, it may be classified as a Class III device, necessitating a more rigorous Premarket Approval (PMA) process.
The next step involves conducting extensive preclinical studies to demonstrate the safety and efficacy of the hydroxyapatite-enhanced biodegradable bone graft. These studies typically include in vitro tests to assess biocompatibility, cytotoxicity, and material properties, as well as in vivo animal studies to evaluate osseointegration, biodegradation rates, and potential adverse effects.
Following successful preclinical trials, developers must design and conduct clinical trials. The FDA's Investigational Device Exemption (IDE) process allows for the use of investigational devices in human clinical studies. These trials are crucial for demonstrating the safety and effectiveness of the novel bone graft material in human subjects.
Throughout the development process, manufacturers must adhere to Good Manufacturing Practices (GMP) and establish a robust quality management system. This ensures consistency in production and helps maintain the quality and safety of the final product.
The regulatory submission package must include comprehensive data from preclinical and clinical studies, detailed information on the manufacturing process, and a thorough risk assessment. Regulatory agencies will review this package, often engaging in a dialogue with the developers to address any concerns or request additional information.
Post-market surveillance is a critical component of the regulatory pathway. Manufacturers are required to monitor the performance of their bone graft materials after market approval, reporting any adverse events or unexpected outcomes to the regulatory authorities.
Lastly, it's important to note that regulatory requirements may vary between different countries and regions. Developers aiming for global markets must consider these variations and potentially pursue multiple regulatory approvals simultaneously to ensure widespread availability of their innovative bone graft materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!