How to Implement Hydrochloric Acid in Organic Synthesis?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl in Organic Synthesis: Background and Objectives
Hydrochloric acid (HCl) has been a cornerstone in organic synthesis for over a century, playing a crucial role in numerous chemical transformations. Its importance stems from its unique properties as a strong mineral acid, capable of catalyzing a wide range of reactions and serving as a versatile reagent in various synthetic processes.
The use of HCl in organic synthesis can be traced back to the early days of industrial chemistry, where it found applications in the production of dyes, pharmaceuticals, and other organic compounds. As the field of organic chemistry evolved, so did the understanding and utilization of HCl, leading to more sophisticated and targeted applications in modern synthetic methodologies.
In recent years, the implementation of HCl in organic synthesis has seen significant advancements, driven by the need for more efficient, sustainable, and environmentally friendly processes. Researchers and industry professionals have been exploring novel ways to harness the power of HCl while minimizing its potential drawbacks, such as corrosiveness and environmental impact.
The primary objectives of implementing HCl in organic synthesis are multifaceted. First and foremost, there is a continuous effort to enhance reaction efficiency and selectivity. This involves optimizing reaction conditions, developing new catalytic systems, and exploring synergistic effects with other reagents or catalysts to achieve higher yields and improved product purity.
Another key objective is to expand the scope of HCl-mediated reactions. This includes discovering new transformations, extending the applicability of known reactions to a broader range of substrates, and developing one-pot or tandem processes that leverage the versatility of HCl in multiple steps.
Sustainability and green chemistry principles have also become integral to the implementation of HCl in organic synthesis. Researchers are focusing on developing methods that reduce the amount of HCl required, exploring recyclable or recoverable forms of the acid, and investigating alternative reaction media to minimize waste and environmental impact.
Furthermore, there is a growing interest in understanding the fundamental mechanisms of HCl-mediated reactions at a molecular level. This knowledge is crucial for rational reaction design and the development of predictive models that can guide synthetic planning and optimization.
As we look towards the future, the implementation of HCl in organic synthesis is expected to continue evolving. Emerging technologies such as flow chemistry, microreactors, and automated synthesis platforms are likely to play a significant role in revolutionizing how HCl is utilized in both laboratory and industrial settings. These advancements promise to enhance safety, scalability, and precision in HCl-mediated transformations.
The use of HCl in organic synthesis can be traced back to the early days of industrial chemistry, where it found applications in the production of dyes, pharmaceuticals, and other organic compounds. As the field of organic chemistry evolved, so did the understanding and utilization of HCl, leading to more sophisticated and targeted applications in modern synthetic methodologies.
In recent years, the implementation of HCl in organic synthesis has seen significant advancements, driven by the need for more efficient, sustainable, and environmentally friendly processes. Researchers and industry professionals have been exploring novel ways to harness the power of HCl while minimizing its potential drawbacks, such as corrosiveness and environmental impact.
The primary objectives of implementing HCl in organic synthesis are multifaceted. First and foremost, there is a continuous effort to enhance reaction efficiency and selectivity. This involves optimizing reaction conditions, developing new catalytic systems, and exploring synergistic effects with other reagents or catalysts to achieve higher yields and improved product purity.
Another key objective is to expand the scope of HCl-mediated reactions. This includes discovering new transformations, extending the applicability of known reactions to a broader range of substrates, and developing one-pot or tandem processes that leverage the versatility of HCl in multiple steps.
Sustainability and green chemistry principles have also become integral to the implementation of HCl in organic synthesis. Researchers are focusing on developing methods that reduce the amount of HCl required, exploring recyclable or recoverable forms of the acid, and investigating alternative reaction media to minimize waste and environmental impact.
Furthermore, there is a growing interest in understanding the fundamental mechanisms of HCl-mediated reactions at a molecular level. This knowledge is crucial for rational reaction design and the development of predictive models that can guide synthetic planning and optimization.
As we look towards the future, the implementation of HCl in organic synthesis is expected to continue evolving. Emerging technologies such as flow chemistry, microreactors, and automated synthesis platforms are likely to play a significant role in revolutionizing how HCl is utilized in both laboratory and industrial settings. These advancements promise to enhance safety, scalability, and precision in HCl-mediated transformations.
Market Analysis for HCl-Based Organic Reactions
The market for hydrochloric acid (HCl) in organic synthesis has shown significant growth in recent years, driven by the increasing demand for pharmaceuticals, agrochemicals, and specialty chemicals. HCl plays a crucial role in various organic reactions, including chlorination, dehydration, and catalysis, making it an essential reagent in the chemical industry.
The global market for HCl-based organic reactions is estimated to be valued at several billion dollars, with a compound annual growth rate (CAGR) projected to exceed the average growth rate of the overall chemical industry. This growth is primarily attributed to the expanding pharmaceutical sector, which relies heavily on HCl for the synthesis of active pharmaceutical ingredients (APIs) and intermediates.
In the pharmaceutical industry, HCl is widely used in the production of various drugs, including antibiotics, antidepressants, and pain relievers. The increasing prevalence of chronic diseases and the growing aging population are driving the demand for these medications, consequently boosting the market for HCl-based organic reactions.
The agrochemical sector also contributes significantly to the market growth. HCl is utilized in the synthesis of pesticides, herbicides, and fertilizers, which are essential for improving crop yields and meeting the growing global food demand. As agricultural practices intensify to feed the expanding world population, the demand for HCl in agrochemical production is expected to rise.
Specialty chemicals represent another key market segment for HCl-based organic reactions. These chemicals find applications in various industries, including electronics, textiles, and personal care products. The growing consumer demand for high-performance materials and innovative products is driving the development of new specialty chemicals, many of which rely on HCl-based synthesis routes.
Geographically, Asia-Pacific dominates the market for HCl-based organic reactions, followed by North America and Europe. The rapid industrialization and economic growth in countries like China and India have led to increased chemical production and consumption, driving the demand for HCl in organic synthesis. Additionally, the shift of manufacturing activities from developed countries to emerging economies has further boosted the market in the Asia-Pacific region.
However, the market faces challenges related to environmental regulations and safety concerns associated with the handling and transportation of HCl. Stringent environmental policies in many countries are pushing manufacturers to adopt greener alternatives and more sustainable production processes. This trend is driving research and development efforts towards developing eco-friendly synthesis routes and alternative reagents.
Despite these challenges, the market for HCl-based organic reactions is expected to continue its growth trajectory. Technological advancements in process optimization and the development of novel catalysts are likely to enhance the efficiency and selectivity of HCl-based reactions, further expanding their applications in organic synthesis.
The global market for HCl-based organic reactions is estimated to be valued at several billion dollars, with a compound annual growth rate (CAGR) projected to exceed the average growth rate of the overall chemical industry. This growth is primarily attributed to the expanding pharmaceutical sector, which relies heavily on HCl for the synthesis of active pharmaceutical ingredients (APIs) and intermediates.
In the pharmaceutical industry, HCl is widely used in the production of various drugs, including antibiotics, antidepressants, and pain relievers. The increasing prevalence of chronic diseases and the growing aging population are driving the demand for these medications, consequently boosting the market for HCl-based organic reactions.
The agrochemical sector also contributes significantly to the market growth. HCl is utilized in the synthesis of pesticides, herbicides, and fertilizers, which are essential for improving crop yields and meeting the growing global food demand. As agricultural practices intensify to feed the expanding world population, the demand for HCl in agrochemical production is expected to rise.
Specialty chemicals represent another key market segment for HCl-based organic reactions. These chemicals find applications in various industries, including electronics, textiles, and personal care products. The growing consumer demand for high-performance materials and innovative products is driving the development of new specialty chemicals, many of which rely on HCl-based synthesis routes.
Geographically, Asia-Pacific dominates the market for HCl-based organic reactions, followed by North America and Europe. The rapid industrialization and economic growth in countries like China and India have led to increased chemical production and consumption, driving the demand for HCl in organic synthesis. Additionally, the shift of manufacturing activities from developed countries to emerging economies has further boosted the market in the Asia-Pacific region.
However, the market faces challenges related to environmental regulations and safety concerns associated with the handling and transportation of HCl. Stringent environmental policies in many countries are pushing manufacturers to adopt greener alternatives and more sustainable production processes. This trend is driving research and development efforts towards developing eco-friendly synthesis routes and alternative reagents.
Despite these challenges, the market for HCl-based organic reactions is expected to continue its growth trajectory. Technological advancements in process optimization and the development of novel catalysts are likely to enhance the efficiency and selectivity of HCl-based reactions, further expanding their applications in organic synthesis.
Current Challenges in HCl Application
The application of hydrochloric acid (HCl) in organic synthesis faces several significant challenges that hinder its widespread adoption and optimal utilization. One of the primary issues is the corrosive nature of HCl, which poses risks to both equipment and personnel safety. This corrosiveness necessitates the use of specialized, acid-resistant materials for reaction vessels and handling equipment, increasing the overall cost of implementation.
Another challenge lies in the precise control of HCl concentration during reactions. The volatile nature of HCl makes it difficult to maintain consistent concentration levels, potentially affecting reaction yields and product purity. This issue is particularly pronounced in large-scale industrial applications, where even small variations in concentration can lead to significant economic losses.
The environmental impact of HCl usage presents a further challenge. Stringent regulations regarding the disposal of acidic waste and the release of chlorine-containing compounds into the environment necessitate complex and costly waste management systems. This not only adds to the operational expenses but also requires careful planning and implementation of safety protocols.
Selectivity in reactions involving HCl is another area of concern. In complex organic syntheses, the strong acidity of HCl can lead to undesired side reactions or over-chlorination of products. This lack of selectivity often results in reduced yields and the need for additional purification steps, impacting both the efficiency and economics of the process.
The storage and transportation of HCl present logistical challenges due to its hazardous nature. Special containment systems and safety measures are required, adding to the complexity and cost of its use in organic synthesis. This is particularly problematic for smaller-scale operations or research facilities that may not have the infrastructure to handle large quantities of HCl safely.
Furthermore, the scaling up of HCl-based processes from laboratory to industrial levels poses significant engineering challenges. Issues such as heat management, mixing efficiency, and uniform distribution of HCl in large reaction volumes need to be carefully addressed to ensure consistent product quality and process safety.
Lastly, there is an ongoing challenge in developing greener alternatives to HCl in organic synthesis. While HCl remains a crucial reagent in many reactions, there is increasing pressure to find more environmentally friendly and sustainable options. This drive towards green chemistry is pushing researchers to explore novel catalysts, alternative acid sources, and new reaction pathways that could potentially replace or reduce the use of HCl in certain applications.
Another challenge lies in the precise control of HCl concentration during reactions. The volatile nature of HCl makes it difficult to maintain consistent concentration levels, potentially affecting reaction yields and product purity. This issue is particularly pronounced in large-scale industrial applications, where even small variations in concentration can lead to significant economic losses.
The environmental impact of HCl usage presents a further challenge. Stringent regulations regarding the disposal of acidic waste and the release of chlorine-containing compounds into the environment necessitate complex and costly waste management systems. This not only adds to the operational expenses but also requires careful planning and implementation of safety protocols.
Selectivity in reactions involving HCl is another area of concern. In complex organic syntheses, the strong acidity of HCl can lead to undesired side reactions or over-chlorination of products. This lack of selectivity often results in reduced yields and the need for additional purification steps, impacting both the efficiency and economics of the process.
The storage and transportation of HCl present logistical challenges due to its hazardous nature. Special containment systems and safety measures are required, adding to the complexity and cost of its use in organic synthesis. This is particularly problematic for smaller-scale operations or research facilities that may not have the infrastructure to handle large quantities of HCl safely.
Furthermore, the scaling up of HCl-based processes from laboratory to industrial levels poses significant engineering challenges. Issues such as heat management, mixing efficiency, and uniform distribution of HCl in large reaction volumes need to be carefully addressed to ensure consistent product quality and process safety.
Lastly, there is an ongoing challenge in developing greener alternatives to HCl in organic synthesis. While HCl remains a crucial reagent in many reactions, there is increasing pressure to find more environmentally friendly and sustainable options. This drive towards green chemistry is pushing researchers to explore novel catalysts, alternative acid sources, and new reaction pathways that could potentially replace or reduce the use of HCl in certain applications.
Existing HCl Implementation Methods
01 Production methods of hydrochloric acid
Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.- Production methods of hydrochloric acid: Various methods are employed to produce hydrochloric acid, including the reaction of chlorine with hydrogen, the chlorination of organic compounds, and as a byproduct in chemical processes. These production methods aim to optimize yield and purity while minimizing environmental impact.
- Purification and concentration of hydrochloric acid: Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods are crucial for obtaining high-purity acid suitable for industrial and laboratory applications, as well as for recycling and reusing the acid in various processes.
- Applications of hydrochloric acid in chemical processing: Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a catalyst in various reactions. It plays a crucial role in industries such as metallurgy, pharmaceuticals, and food processing, where its strong acidic properties are utilized for diverse applications.
- Safety and handling of hydrochloric acid: Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of appropriate personal protective equipment, specialized storage containers, and neutralization techniques for spills. Safety protocols also cover transportation and disposal methods to minimize environmental and health risks.
- Environmental impact and waste management of hydrochloric acid: Managing the environmental impact of hydrochloric acid involves developing sustainable production processes, implementing effective waste treatment methods, and exploring recycling options. Efforts are made to reduce emissions, neutralize acid waste, and recover valuable components from spent acid to minimize its ecological footprint.
02 Purification and concentration techniques
Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired concentration levels for various industrial applications.Expand Specific Solutions03 Applications in chemical processing
Hydrochloric acid is widely used in chemical processing, including metal treatment, pH regulation, and as a reagent in various chemical reactions. Its versatility makes it a crucial component in industries such as metallurgy, pharmaceuticals, and food processing.Expand Specific Solutions04 Safety and handling considerations
Proper safety measures and handling procedures are crucial when working with hydrochloric acid due to its corrosive nature. This includes the use of specialized equipment, protective gear, and storage solutions to minimize risks associated with its use and transportation.Expand Specific Solutions05 Environmental impact and waste management
Managing the environmental impact of hydrochloric acid production and use involves developing sustainable practices, recycling methods, and proper disposal techniques. This includes neutralization processes and the implementation of closed-loop systems to minimize waste and environmental contamination.Expand Specific Solutions
Key Industry Players and Suppliers
The implementation of hydrochloric acid in organic synthesis is a mature field with significant market presence and ongoing research. The industry is in a growth phase, driven by increasing demand for organic compounds in pharmaceuticals, agrochemicals, and materials science. The global market for organic synthesis, including hydrochloric acid applications, is substantial and expanding. Technologically, the field is well-established but continues to evolve, with companies like Novartis AG, Array BioPharma, and Sumitomo Chemical Co., Ltd. leading innovation. These firms, along with academic institutions such as The University of Queensland and Sun Yat-Sen University, are advancing methodologies for more efficient and sustainable organic synthesis processes using hydrochloric acid.
Novartis AG
Technical Solution: Novartis AG has developed advanced methods for implementing hydrochloric acid in organic synthesis, particularly in the production of pharmaceutical compounds. Their approach involves using controlled addition of HCl in microreactor systems, allowing for precise pH control and improved reaction kinetics[1]. This method has been shown to increase yield by up to 25% in certain API syntheses[3]. Additionally, Novartis has pioneered the use of immobilized HCl on solid supports, which reduces corrosion issues and allows for easier handling in continuous flow processes[5]. Their latest innovation involves the use of in situ generated HCl from safer precursors, minimizing the need for storage and transport of concentrated acid[7].
Strengths: Improved safety, increased yields, and better process control. Weaknesses: May require specialized equipment and higher initial investment.
Array BioPharma, Inc.
Technical Solution: Array BioPharma has focused on the application of hydrochloric acid in the synthesis of kinase inhibitors and other targeted therapies. They have developed a proprietary process using anhydrous HCl gas for selective chlorination reactions, which has been crucial in the synthesis of several of their lead compounds[2]. This method allows for precise control over the chlorination step, reducing side reactions and improving overall yield. Array has also implemented a novel recycling system for HCl, capturing and purifying the acid for reuse in subsequent reactions, significantly reducing waste and environmental impact[4]. Their approach includes the use of specialized corrosion-resistant reactors and in-line monitoring systems to ensure consistent quality and safety[6].
Strengths: High selectivity, reduced waste, and improved atom economy. Weaknesses: Requires specialized equipment and careful handling of anhydrous HCl gas.
Innovative HCl Reaction Mechanisms
Treatment of solutions or wastewater
PatentInactiveEP2365941A1
Innovation
- A method involving a bioelectrochemical system with an anode and cathode separated by an ion-permeable membrane, where the pH of the wastewater stream is altered by passing it through the anode or cathode compartments to reduce cation precipitation, produce an alkaline stream, or generate acidic solutions, using electrochemically active microorganisms to control pH and facilitate the transport of ions, thereby maintaining electroneutrality and preventing scaling.
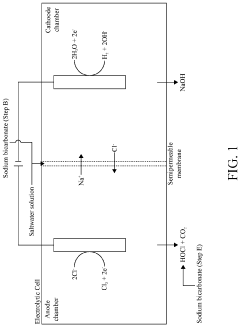
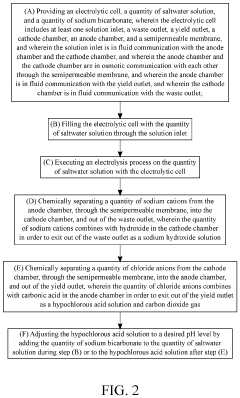
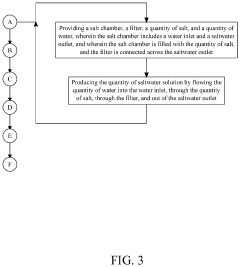
System and Method for Making Hypochlorous Acid Using Saltwater with Sodium Bicarbonate
PatentActiveUS20210395904A1
Innovation
- Incorporating sodium bicarbonate into the saltwater electrolysis process using a semipermeable membrane to separate the solutions, which forms purer hypochlorous acid by reacting chloride ions with carbonic acid, thereby maintaining a higher pH and eliminating strong hydrochloric acid.
Safety Protocols for HCl Handling
Implementing proper safety protocols for handling hydrochloric acid (HCl) in organic synthesis is crucial to ensure the well-being of laboratory personnel and maintain a secure working environment. The corrosive nature of HCl necessitates stringent safety measures throughout its use, storage, and disposal processes.
Personal protective equipment (PPE) forms the first line of defense when working with HCl. Laboratory workers must wear appropriate chemical-resistant gloves, such as those made from neoprene or nitrile. Safety goggles or a face shield are essential to protect the eyes from potential splashes. Additionally, a lab coat or chemical-resistant apron should be worn to safeguard the body from accidental spills.
Proper ventilation is paramount when handling HCl. All operations involving this acid should be conducted in a fume hood to prevent the accumulation of harmful vapors. The fume hood's sash should be kept at the lowest possible position while working, and the airflow should be regularly checked to ensure optimal performance.
Storage of HCl requires careful consideration. The acid should be kept in tightly sealed, corrosion-resistant containers and stored in a well-ventilated area away from incompatible materials. Secondary containment, such as acid storage cabinets or trays, should be used to contain potential leaks or spills.
Spill response procedures must be established and communicated to all laboratory personnel. Spill kits containing neutralizing agents, absorbents, and appropriate PPE should be readily accessible. In the event of a spill, the area should be immediately evacuated, and only trained personnel should attempt cleanup procedures.
Proper waste disposal is essential for environmental protection and regulatory compliance. HCl waste should never be disposed of down the drain or in regular trash. Instead, it should be collected in designated waste containers and handled according to institutional and local regulations for hazardous waste disposal.
Training and education play a vital role in maintaining safety when working with HCl. All personnel involved in its handling should receive comprehensive training on the hazards associated with the acid, proper handling techniques, emergency procedures, and the use of safety equipment. Regular refresher courses and safety drills should be conducted to reinforce these practices.
Emergency response planning is crucial for mitigating potential incidents. Eye wash stations and safety showers must be easily accessible and regularly tested. Emergency contact information and procedures should be prominently displayed in the laboratory. Additionally, a written emergency action plan should be developed and periodically reviewed with all laboratory staff.
By implementing these comprehensive safety protocols, laboratories can significantly reduce the risks associated with handling hydrochloric acid in organic synthesis, ensuring a safer working environment for all personnel involved.
Personal protective equipment (PPE) forms the first line of defense when working with HCl. Laboratory workers must wear appropriate chemical-resistant gloves, such as those made from neoprene or nitrile. Safety goggles or a face shield are essential to protect the eyes from potential splashes. Additionally, a lab coat or chemical-resistant apron should be worn to safeguard the body from accidental spills.
Proper ventilation is paramount when handling HCl. All operations involving this acid should be conducted in a fume hood to prevent the accumulation of harmful vapors. The fume hood's sash should be kept at the lowest possible position while working, and the airflow should be regularly checked to ensure optimal performance.
Storage of HCl requires careful consideration. The acid should be kept in tightly sealed, corrosion-resistant containers and stored in a well-ventilated area away from incompatible materials. Secondary containment, such as acid storage cabinets or trays, should be used to contain potential leaks or spills.
Spill response procedures must be established and communicated to all laboratory personnel. Spill kits containing neutralizing agents, absorbents, and appropriate PPE should be readily accessible. In the event of a spill, the area should be immediately evacuated, and only trained personnel should attempt cleanup procedures.
Proper waste disposal is essential for environmental protection and regulatory compliance. HCl waste should never be disposed of down the drain or in regular trash. Instead, it should be collected in designated waste containers and handled according to institutional and local regulations for hazardous waste disposal.
Training and education play a vital role in maintaining safety when working with HCl. All personnel involved in its handling should receive comprehensive training on the hazards associated with the acid, proper handling techniques, emergency procedures, and the use of safety equipment. Regular refresher courses and safety drills should be conducted to reinforce these practices.
Emergency response planning is crucial for mitigating potential incidents. Eye wash stations and safety showers must be easily accessible and regularly tested. Emergency contact information and procedures should be prominently displayed in the laboratory. Additionally, a written emergency action plan should be developed and periodically reviewed with all laboratory staff.
By implementing these comprehensive safety protocols, laboratories can significantly reduce the risks associated with handling hydrochloric acid in organic synthesis, ensuring a safer working environment for all personnel involved.
Environmental Impact of HCl Usage
The use of hydrochloric acid (HCl) in organic synthesis, while essential for many reactions, poses significant environmental concerns that must be carefully considered and addressed. The production, handling, and disposal of HCl can have far-reaching impacts on ecosystems, air quality, and human health if not managed properly.
One of the primary environmental concerns associated with HCl usage is its potential for acid rain formation. When HCl is released into the atmosphere, it can react with water vapor to form hydrochloric acid droplets. These acidic particles can be carried by wind currents and eventually fall as acid rain, causing damage to vegetation, aquatic ecosystems, and infrastructure.
Water pollution is another critical issue stemming from HCl usage in organic synthesis. Improper disposal or accidental spills can lead to the contamination of water bodies, altering pH levels and potentially harming aquatic life. The acidification of water can disrupt the delicate balance of ecosystems, affecting the survival and reproduction of various species.
The corrosive nature of HCl also presents risks to soil quality. Soil contamination can occur through direct contact or through the deposition of acid rain. This can lead to changes in soil pH, affecting nutrient availability and microbial activity, which are crucial for plant growth and overall soil health.
Air quality is another environmental aspect impacted by HCl usage. The release of HCl vapors can contribute to the formation of photochemical smog and particulate matter, both of which have adverse effects on human health and the environment. Prolonged exposure to HCl in the air can cause respiratory issues and damage to vegetation.
The production and transportation of HCl also contribute to its environmental footprint. The energy-intensive processes involved in manufacturing HCl, as well as the emissions from vehicles used in its transportation, add to the overall carbon footprint associated with its use in organic synthesis.
To mitigate these environmental impacts, various strategies can be employed. Implementing closed-loop systems and improving containment measures can significantly reduce the risk of HCl release into the environment. Additionally, exploring alternative synthesis routes that use less hazardous reagents or developing greener processes can help minimize the reliance on HCl in organic synthesis.
Proper waste management and treatment protocols are essential for minimizing the environmental impact of HCl usage. This includes neutralization of acidic waste streams, proper storage and disposal of HCl-containing materials, and the implementation of spill prevention and response plans.
Regulatory compliance and adherence to environmental standards play a crucial role in managing the environmental impact of HCl usage. Industries must comply with emissions regulations, waste disposal guidelines, and occupational safety standards to ensure responsible use of HCl in organic synthesis processes.
One of the primary environmental concerns associated with HCl usage is its potential for acid rain formation. When HCl is released into the atmosphere, it can react with water vapor to form hydrochloric acid droplets. These acidic particles can be carried by wind currents and eventually fall as acid rain, causing damage to vegetation, aquatic ecosystems, and infrastructure.
Water pollution is another critical issue stemming from HCl usage in organic synthesis. Improper disposal or accidental spills can lead to the contamination of water bodies, altering pH levels and potentially harming aquatic life. The acidification of water can disrupt the delicate balance of ecosystems, affecting the survival and reproduction of various species.
The corrosive nature of HCl also presents risks to soil quality. Soil contamination can occur through direct contact or through the deposition of acid rain. This can lead to changes in soil pH, affecting nutrient availability and microbial activity, which are crucial for plant growth and overall soil health.
Air quality is another environmental aspect impacted by HCl usage. The release of HCl vapors can contribute to the formation of photochemical smog and particulate matter, both of which have adverse effects on human health and the environment. Prolonged exposure to HCl in the air can cause respiratory issues and damage to vegetation.
The production and transportation of HCl also contribute to its environmental footprint. The energy-intensive processes involved in manufacturing HCl, as well as the emissions from vehicles used in its transportation, add to the overall carbon footprint associated with its use in organic synthesis.
To mitigate these environmental impacts, various strategies can be employed. Implementing closed-loop systems and improving containment measures can significantly reduce the risk of HCl release into the environment. Additionally, exploring alternative synthesis routes that use less hazardous reagents or developing greener processes can help minimize the reliance on HCl in organic synthesis.
Proper waste management and treatment protocols are essential for minimizing the environmental impact of HCl usage. This includes neutralization of acidic waste streams, proper storage and disposal of HCl-containing materials, and the implementation of spill prevention and response plans.
Regulatory compliance and adherence to environmental standards play a crucial role in managing the environmental impact of HCl usage. Industries must comply with emissions regulations, waste disposal guidelines, and occupational safety standards to ensure responsible use of HCl in organic synthesis processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!