How To Test Lithium Hydroxide For Contaminant Presence
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Hydroxide Testing Background and Objectives
Lithium hydroxide (LiOH) has emerged as a critical material in the global transition toward clean energy, particularly in the production of high-performance lithium-ion batteries for electric vehicles and energy storage systems. The historical development of lithium hydroxide testing methodologies can be traced back to traditional analytical chemistry techniques, which have evolved significantly over the past decades to meet the increasingly stringent quality requirements of modern industrial applications.
The growing demand for high-purity lithium hydroxide has intensified the need for sophisticated contaminant detection methods. Impurities such as sodium, potassium, calcium, magnesium, iron, and various heavy metals can significantly impact battery performance, safety, and longevity. Even trace amounts of these contaminants can lead to reduced energy density, accelerated degradation, and potential safety hazards in lithium-ion batteries.
The technological evolution in lithium hydroxide testing has progressed from basic wet chemistry methods to advanced instrumental techniques. Early testing relied primarily on gravimetric and titrimetric analyses, which provided limited sensitivity and specificity. The introduction of atomic absorption spectroscopy (AAS) in the mid-20th century marked a significant advancement, enabling more precise quantification of metallic impurities.
Recent decades have witnessed the integration of more sophisticated analytical technologies, including inductively coupled plasma mass spectrometry (ICP-MS), ion chromatography (IC), and X-ray fluorescence (XRF) spectroscopy. These methods offer substantially improved detection limits, often in the parts-per-billion range, allowing for comprehensive impurity profiling of lithium hydroxide samples.
The primary objectives of modern lithium hydroxide testing protocols are multifaceted. First, they aim to ensure compliance with increasingly stringent industry specifications and regulatory standards. Second, they seek to provide comprehensive characterization of contaminant profiles to support quality control and process optimization in manufacturing settings. Third, they strive to enable real-time or near-real-time monitoring capabilities to facilitate rapid decision-making in production environments.
Future technological trends in this domain are moving toward non-destructive, in-line testing methodologies that can be integrated directly into production processes. Additionally, there is growing interest in developing portable, field-deployable testing solutions that can provide immediate results without requiring sophisticated laboratory infrastructure. These advancements align with the broader industry goals of improving efficiency, reducing costs, and enhancing quality assurance in the lithium battery supply chain.
The development of standardized testing protocols represents another critical objective, as harmonized methodologies would facilitate more consistent quality assessment across the global lithium hydroxide market, ultimately supporting the continued growth and reliability of clean energy technologies.
The growing demand for high-purity lithium hydroxide has intensified the need for sophisticated contaminant detection methods. Impurities such as sodium, potassium, calcium, magnesium, iron, and various heavy metals can significantly impact battery performance, safety, and longevity. Even trace amounts of these contaminants can lead to reduced energy density, accelerated degradation, and potential safety hazards in lithium-ion batteries.
The technological evolution in lithium hydroxide testing has progressed from basic wet chemistry methods to advanced instrumental techniques. Early testing relied primarily on gravimetric and titrimetric analyses, which provided limited sensitivity and specificity. The introduction of atomic absorption spectroscopy (AAS) in the mid-20th century marked a significant advancement, enabling more precise quantification of metallic impurities.
Recent decades have witnessed the integration of more sophisticated analytical technologies, including inductively coupled plasma mass spectrometry (ICP-MS), ion chromatography (IC), and X-ray fluorescence (XRF) spectroscopy. These methods offer substantially improved detection limits, often in the parts-per-billion range, allowing for comprehensive impurity profiling of lithium hydroxide samples.
The primary objectives of modern lithium hydroxide testing protocols are multifaceted. First, they aim to ensure compliance with increasingly stringent industry specifications and regulatory standards. Second, they seek to provide comprehensive characterization of contaminant profiles to support quality control and process optimization in manufacturing settings. Third, they strive to enable real-time or near-real-time monitoring capabilities to facilitate rapid decision-making in production environments.
Future technological trends in this domain are moving toward non-destructive, in-line testing methodologies that can be integrated directly into production processes. Additionally, there is growing interest in developing portable, field-deployable testing solutions that can provide immediate results without requiring sophisticated laboratory infrastructure. These advancements align with the broader industry goals of improving efficiency, reducing costs, and enhancing quality assurance in the lithium battery supply chain.
The development of standardized testing protocols represents another critical objective, as harmonized methodologies would facilitate more consistent quality assessment across the global lithium hydroxide market, ultimately supporting the continued growth and reliability of clean energy technologies.
Market Demand Analysis for High-Purity Lithium Hydroxide
The global market for high-purity lithium hydroxide has experienced exponential growth in recent years, primarily driven by the rapid expansion of the electric vehicle (EV) industry. As battery technology advances, the demand for battery-grade lithium hydroxide with minimal contaminants has become increasingly critical. Market research indicates that the global lithium hydroxide market value reached approximately $2.3 billion in 2022 and is projected to grow at a compound annual growth rate of 16.2% through 2030.
The EV sector represents the largest consumer of high-purity lithium hydroxide, accounting for over 65% of total demand. This is attributed to the shift toward nickel-rich cathode materials in lithium-ion batteries, which require lithium hydroxide rather than lithium carbonate as a precursor. Major automakers have announced ambitious electrification targets, with some planning to achieve 100% electric vehicle production by 2035, further accelerating demand.
Battery manufacturers are increasingly implementing stringent specifications for lithium hydroxide purity, typically requiring 99.5% purity or higher with strict limits on specific contaminants such as sodium, potassium, calcium, and heavy metals. These impurities, even at parts-per-million levels, can significantly impact battery performance, safety, and longevity. Consequently, reliable testing methods for contaminant detection have become essential quality control measures.
The energy storage system (ESS) market represents another growing segment for high-purity lithium hydroxide, expected to expand at a rate of 20% annually as renewable energy integration accelerates globally. Grid-scale storage applications are particularly sensitive to contaminants that could compromise system reliability over their expected 10-20 year operational lifespan.
Regional analysis reveals that Asia-Pacific dominates the market demand, with China accounting for approximately 48% of global consumption. However, significant growth is anticipated in North America and Europe as domestic battery production capacity expands to reduce supply chain vulnerabilities. This regionalization trend is creating new market opportunities for localized lithium hydroxide production and testing services.
Price sensitivity analysis indicates that while high-purity lithium hydroxide commands a premium of 15-25% over standard grades, manufacturers are willing to pay this premium due to the critical impact of contaminants on end-product performance. This has created a specialized market segment focused on advanced testing and quality assurance services, estimated to be worth $340 million annually.
Industry surveys reveal that 78% of battery manufacturers consider contaminant testing capabilities a critical factor when selecting lithium hydroxide suppliers. This market pressure has accelerated innovation in testing methodologies, with demand for faster, more accurate, and comprehensive contaminant detection solutions growing at twice the rate of the overall lithium hydroxide market.
The EV sector represents the largest consumer of high-purity lithium hydroxide, accounting for over 65% of total demand. This is attributed to the shift toward nickel-rich cathode materials in lithium-ion batteries, which require lithium hydroxide rather than lithium carbonate as a precursor. Major automakers have announced ambitious electrification targets, with some planning to achieve 100% electric vehicle production by 2035, further accelerating demand.
Battery manufacturers are increasingly implementing stringent specifications for lithium hydroxide purity, typically requiring 99.5% purity or higher with strict limits on specific contaminants such as sodium, potassium, calcium, and heavy metals. These impurities, even at parts-per-million levels, can significantly impact battery performance, safety, and longevity. Consequently, reliable testing methods for contaminant detection have become essential quality control measures.
The energy storage system (ESS) market represents another growing segment for high-purity lithium hydroxide, expected to expand at a rate of 20% annually as renewable energy integration accelerates globally. Grid-scale storage applications are particularly sensitive to contaminants that could compromise system reliability over their expected 10-20 year operational lifespan.
Regional analysis reveals that Asia-Pacific dominates the market demand, with China accounting for approximately 48% of global consumption. However, significant growth is anticipated in North America and Europe as domestic battery production capacity expands to reduce supply chain vulnerabilities. This regionalization trend is creating new market opportunities for localized lithium hydroxide production and testing services.
Price sensitivity analysis indicates that while high-purity lithium hydroxide commands a premium of 15-25% over standard grades, manufacturers are willing to pay this premium due to the critical impact of contaminants on end-product performance. This has created a specialized market segment focused on advanced testing and quality assurance services, estimated to be worth $340 million annually.
Industry surveys reveal that 78% of battery manufacturers consider contaminant testing capabilities a critical factor when selecting lithium hydroxide suppliers. This market pressure has accelerated innovation in testing methodologies, with demand for faster, more accurate, and comprehensive contaminant detection solutions growing at twice the rate of the overall lithium hydroxide market.
Current Testing Methods and Technical Challenges
The current landscape of lithium hydroxide testing methodologies encompasses several established techniques, each with specific applications and limitations. Titration methods, particularly acid-base titration, represent the traditional approach for determining lithium hydroxide purity. While cost-effective and accessible, these methods often lack the sensitivity required to detect trace contaminants at parts per million (ppm) levels, which is increasingly critical in high-performance battery applications.
Spectroscopic techniques have gained prominence, with Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Atomic Absorption Spectroscopy (AAS) offering detection limits in the parts per billion (ppb) range. These methods excel at identifying metallic impurities such as sodium, potassium, calcium, and transition metals that significantly impact battery performance. However, the instrumentation costs remain prohibitive for smaller operations, and sample preparation complexity introduces potential for procedural errors.
X-ray fluorescence (XRF) provides rapid, non-destructive analysis of elemental composition but struggles with lighter elements and trace contaminants below approximately 10 ppm. This limitation restricts its utility for comprehensive quality control in premium-grade lithium hydroxide production.
Chromatographic methods, particularly ion chromatography, have emerged as powerful tools for detecting ionic impurities. These techniques offer excellent sensitivity for sulfates, chlorides, and other anions that can compromise battery electrolyte stability. The primary challenges include lengthy analysis times and the need for specialized columns for different contaminant classes.
Technical challenges in lithium hydroxide testing extend beyond methodological limitations. Sample heterogeneity presents a significant obstacle, as contaminants may not be uniformly distributed throughout production batches. This necessitates robust sampling protocols that many current standards fail to adequately address.
The hygroscopic nature of lithium hydroxide compounds further complicates testing procedures, as moisture absorption during handling can dilute samples and introduce measurement errors. Specialized environmental controls during testing are required but not universally implemented.
Emerging battery technologies demand increasingly stringent purity requirements, with some applications requiring detection of organic contaminants at sub-ppm levels. Current testing regimes often focus predominantly on inorganic impurities, creating a potential blind spot for organic compounds that may impact long-term battery performance.
Standardization remains problematic across the industry, with different regions and organizations employing varied testing protocols and acceptance criteria. This hampers global trade and technology transfer, as test results from different facilities may not be directly comparable without extensive cross-validation efforts.
Spectroscopic techniques have gained prominence, with Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Atomic Absorption Spectroscopy (AAS) offering detection limits in the parts per billion (ppb) range. These methods excel at identifying metallic impurities such as sodium, potassium, calcium, and transition metals that significantly impact battery performance. However, the instrumentation costs remain prohibitive for smaller operations, and sample preparation complexity introduces potential for procedural errors.
X-ray fluorescence (XRF) provides rapid, non-destructive analysis of elemental composition but struggles with lighter elements and trace contaminants below approximately 10 ppm. This limitation restricts its utility for comprehensive quality control in premium-grade lithium hydroxide production.
Chromatographic methods, particularly ion chromatography, have emerged as powerful tools for detecting ionic impurities. These techniques offer excellent sensitivity for sulfates, chlorides, and other anions that can compromise battery electrolyte stability. The primary challenges include lengthy analysis times and the need for specialized columns for different contaminant classes.
Technical challenges in lithium hydroxide testing extend beyond methodological limitations. Sample heterogeneity presents a significant obstacle, as contaminants may not be uniformly distributed throughout production batches. This necessitates robust sampling protocols that many current standards fail to adequately address.
The hygroscopic nature of lithium hydroxide compounds further complicates testing procedures, as moisture absorption during handling can dilute samples and introduce measurement errors. Specialized environmental controls during testing are required but not universally implemented.
Emerging battery technologies demand increasingly stringent purity requirements, with some applications requiring detection of organic contaminants at sub-ppm levels. Current testing regimes often focus predominantly on inorganic impurities, creating a potential blind spot for organic compounds that may impact long-term battery performance.
Standardization remains problematic across the industry, with different regions and organizations employing varied testing protocols and acceptance criteria. This hampers global trade and technology transfer, as test results from different facilities may not be directly comparable without extensive cross-validation efforts.
Standard Testing Protocols and Methodologies
01 Spectroscopic methods for lithium hydroxide contaminant detection
Various spectroscopic techniques can be employed for detecting contaminants in lithium hydroxide. These methods include infrared spectroscopy, Raman spectroscopy, and mass spectrometry, which can identify specific impurities based on their unique spectral signatures. These techniques offer high sensitivity and can detect trace amounts of contaminants in lithium hydroxide samples, making them valuable for quality control in battery material production.- Spectroscopic methods for lithium hydroxide contaminant detection: Various spectroscopic techniques can be employed for detecting contaminants in lithium hydroxide. These methods include infrared spectroscopy, Raman spectroscopy, and mass spectrometry, which can identify specific contaminant signatures based on their unique spectral patterns. These non-destructive analytical approaches allow for rapid and accurate identification of impurities in lithium hydroxide samples, which is crucial for quality control in battery manufacturing and other applications.
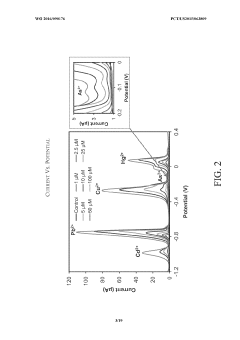
- Electrochemical detection methods for lithium hydroxide purity: Electrochemical techniques provide sensitive methods for detecting contaminants in lithium hydroxide. These approaches utilize electrodes and potential differences to measure impurities based on their electrochemical properties. Techniques such as voltammetry, potentiometry, and impedance spectroscopy can detect trace amounts of metallic and non-metallic contaminants that might affect the performance of lithium hydroxide in battery applications. These methods are particularly valuable for in-line monitoring during production processes.
- Chromatographic separation for contaminant analysis: Chromatographic methods offer powerful separation techniques for identifying contaminants in lithium hydroxide. Ion chromatography, high-performance liquid chromatography (HPLC), and gas chromatography coupled with various detectors can effectively separate and quantify different impurities present in lithium hydroxide samples. These techniques are particularly useful for detecting organic contaminants, transition metals, and other ionic species that might be present at low concentrations but could significantly impact the performance of lithium hydroxide in applications.
- Automated and in-line monitoring systems: Advanced automated systems have been developed for continuous monitoring of contaminants in lithium hydroxide production. These systems integrate multiple detection technologies with artificial intelligence and machine learning algorithms to provide real-time analysis of contaminant levels. In-line monitoring allows for immediate detection of quality issues during manufacturing, enabling prompt corrective actions and ensuring consistent product quality. These systems can detect a wide range of contaminants including metallic impurities, organic compounds, and moisture.
- Sample preparation techniques for enhanced contaminant detection: Specialized sample preparation methods have been developed to improve the sensitivity and accuracy of lithium hydroxide contaminant detection. These techniques include selective extraction procedures, pre-concentration methods, and matrix modification approaches that help isolate contaminants from the lithium hydroxide matrix. Proper sample preparation is crucial for accurate analysis, especially when detecting trace contaminants that might be present at parts-per-billion levels. These methods enhance the performance of subsequent analytical techniques by reducing interference and improving signal-to-noise ratios.
02 Electrochemical detection methods for lithium hydroxide purity
Electrochemical techniques provide effective means for detecting contaminants in lithium hydroxide. These methods involve measuring electrical properties such as conductivity, impedance, or voltammetric responses that change in the presence of impurities. Electrochemical sensors can be designed with specific selectivity toward certain contaminants, allowing for rapid and in-line monitoring of lithium hydroxide quality during production processes.Expand Specific Solutions03 Chromatographic separation for contaminant identification
Chromatographic methods enable the separation and identification of various contaminants in lithium hydroxide samples. Techniques such as ion chromatography, high-performance liquid chromatography (HPLC), and gas chromatography can effectively isolate impurities from the lithium hydroxide matrix. These methods are particularly useful for detecting organic contaminants, metal impurities, and other trace compounds that might affect the performance of lithium hydroxide in battery applications.Expand Specific Solutions04 Automated and in-line monitoring systems
Advanced automated systems have been developed for continuous monitoring of contaminants in lithium hydroxide production. These systems integrate multiple detection technologies with automated sampling and data analysis capabilities. In-line monitoring allows for real-time quality control during manufacturing processes, enabling immediate corrective actions when contaminant levels exceed acceptable thresholds. Such systems improve production efficiency and ensure consistent product quality.Expand Specific Solutions05 Sample preparation techniques for enhanced detection sensitivity
Specialized sample preparation methods can significantly improve the detection of contaminants in lithium hydroxide. These techniques include selective extraction, pre-concentration, derivatization, and matrix separation procedures that enhance the sensitivity and selectivity of subsequent analytical methods. Proper sample preparation is crucial for accurate detection of trace contaminants, particularly when dealing with complex lithium hydroxide matrices or when targeting specific impurities at very low concentrations.Expand Specific Solutions
Key Industry Players in Lithium Testing Equipment
The lithium hydroxide contaminant testing market is in a growth phase, driven by increasing demand for high-purity materials in the electric vehicle battery sector. The global market size is expanding rapidly, projected to reach significant value as battery production scales up worldwide. Technologically, testing methods are evolving from traditional chemical analysis to more sophisticated instrumental techniques. Leading players like LG Energy Solution, LG Chem, and Toyota are investing heavily in quality control systems, while specialized companies such as Hefei Guoxuan and Jiangxi Yunwei New Materials are developing proprietary testing protocols. Research institutions including Dalian Institute of Chemical Physics and Universität Leipzig are advancing novel detection methodologies. The competitive landscape features both established chemical companies and emerging specialized testing solution providers focusing on higher sensitivity and faster throughput capabilities.
LG Chem Ltd.
Technical Solution: LG Chem has developed a comprehensive multi-stage testing protocol for lithium hydroxide contaminant detection that combines several analytical techniques. Their approach utilizes Inductively Coupled Plasma Mass Spectrometry (ICP-MS) for trace metal detection with sensitivity down to parts per billion levels, allowing identification of metallic impurities like iron, copper, and nickel that can significantly impact battery performance. This is complemented by X-ray Diffraction (XRD) analysis to verify crystalline structure purity and identify crystalline contaminants. For organic impurities, LG Chem employs Gas Chromatography-Mass Spectrometry (GC-MS) and High-Performance Liquid Chromatography (HPLC). Their automated sampling systems ensure statistical validity across production batches, with real-time monitoring capabilities that allow for immediate process adjustments when contaminant thresholds are approached.
Strengths: Comprehensive multi-technique approach provides redundant verification and catches diverse contaminant types. Integration with production systems allows for real-time quality control. Weaknesses: Complex testing protocol requires significant capital investment in analytical equipment and trained personnel. Multiple testing stages may increase production time and costs compared to simpler methods.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered a multi-phase lithium hydroxide testing protocol focused on automotive-grade battery applications. Their approach begins with Thermogravimetric Analysis (TGA) to determine moisture content and decomposition characteristics, followed by Atomic Absorption Spectroscopy (AAS) for metallic impurity detection. Toyota's innovation lies in their development of a rapid electrochemical testing method that directly correlates contaminant presence with battery performance metrics. This technique involves creating small-scale test cells using the lithium hydroxide sample and measuring performance parameters like capacity retention and impedance growth. Their protocol also includes Karl Fischer titration for precise water content determination and Fourier Transform Infrared Spectroscopy (FTIR) for identifying organic contaminants. Toyota has integrated these testing methods into a unified quality assurance system that establishes clear pass/fail criteria based on extensive correlation studies between contaminant levels and long-term battery performance in automotive applications.
Strengths: Direct correlation between testing results and actual battery performance provides practical quality metrics beyond simple purity measurements. Comprehensive approach addresses multiple contaminant types with appropriate specialized techniques. Weaknesses: Time-intensive protocol with multiple testing stages may create production bottlenecks. Small-scale test cell approach requires additional materials and processing steps beyond simple chemical analysis.
Advanced Analytical Techniques for Contaminant Detection
Method for analyzing lithium
PatentInactiveJP2009128203A
Innovation
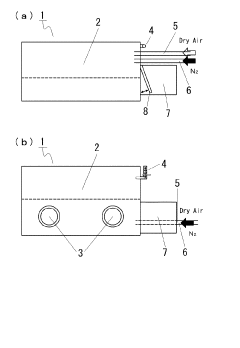
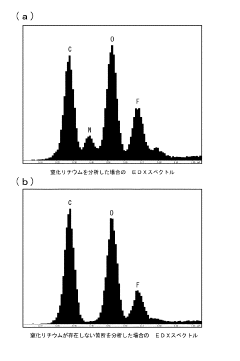
- A method involving the formation of lithium nitride (Li3N) by reacting lithium with nitrogen at room temperature in a high-purity nitrogen atmosphere, followed by analysis using EDX or EPMA to detect the nitrogen component peak, allowing for reliable lithium detection.
Contaminant detection device and method
PatentWO2016090176A1
Innovation
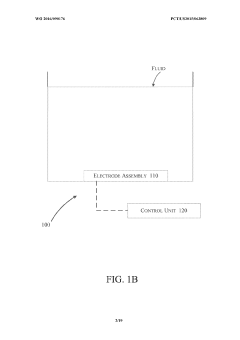
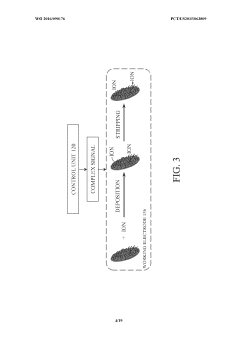
- A device and method utilizing a cysteine functionalized graphene oxide with polypyrrole nanocomposite modified working electrode assembly, coupled with a control unit, to measure contaminant concentrations and pH levels in fluid samples through electrical signals, enhancing sensitivity and accuracy by correlating output signals with contaminant concentrations.
Quality Control Standards and Certification Requirements
Quality control standards for lithium hydroxide testing are governed by several international and industry-specific frameworks. The International Organization for Standardization (ISO) provides comprehensive guidelines through ISO 9001 for quality management systems and ISO/IEC 17025 for testing laboratories. These standards establish the foundation for reliable contaminant detection processes in lithium hydroxide production and processing facilities.
The battery industry, particularly for electric vehicles, has developed stringent specifications through organizations like the Society of Automotive Engineers (SAE) and the International Electrotechnical Commission (IEC). These specifications typically require lithium hydroxide to maintain purity levels exceeding 99.5%, with strict limits on metallic impurities such as sodium, potassium, calcium, and heavy metals, which must often be below 10-50 ppm depending on the application.
Certification requirements vary by region and application sector. In North America, the American Society for Testing and Materials (ASTM) provides standard test methods for lithium compounds, including ASTM E202 for chemical analysis. The European Union enforces REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulations, requiring comprehensive documentation of chemical safety and contaminant profiles.
For battery-grade lithium hydroxide, manufacturers must typically obtain certification from major battery producers or automotive OEMs. These certifications often involve rigorous audit processes examining not only the final product testing protocols but also the entire quality management system, including equipment calibration procedures, staff training, and documentation practices.
The pharmaceutical and food industries impose additional requirements when lithium hydroxide is used in their applications. The United States Pharmacopeia (USP) and Food and Drug Administration (FDA) establish specific limits for heavy metals and other contaminants, requiring validated analytical methods and regular stability testing.
Emerging certification trends include sustainability-focused standards that assess environmental impact of production processes and carbon footprint. Organizations like the Initiative for Responsible Mining Assurance (IRMA) are developing frameworks that incorporate both quality control and environmental sustainability metrics, reflecting the growing importance of ESG (Environmental, Social, and Governance) considerations in the lithium supply chain.
Laboratory accreditation represents another critical component of quality assurance. Testing facilities must typically maintain accreditation to ISO/IEC 17025 standards, demonstrating technical competence through regular proficiency testing and external audits by accreditation bodies such as A2LA in the US or UKAS in the UK.
The battery industry, particularly for electric vehicles, has developed stringent specifications through organizations like the Society of Automotive Engineers (SAE) and the International Electrotechnical Commission (IEC). These specifications typically require lithium hydroxide to maintain purity levels exceeding 99.5%, with strict limits on metallic impurities such as sodium, potassium, calcium, and heavy metals, which must often be below 10-50 ppm depending on the application.
Certification requirements vary by region and application sector. In North America, the American Society for Testing and Materials (ASTM) provides standard test methods for lithium compounds, including ASTM E202 for chemical analysis. The European Union enforces REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulations, requiring comprehensive documentation of chemical safety and contaminant profiles.
For battery-grade lithium hydroxide, manufacturers must typically obtain certification from major battery producers or automotive OEMs. These certifications often involve rigorous audit processes examining not only the final product testing protocols but also the entire quality management system, including equipment calibration procedures, staff training, and documentation practices.
The pharmaceutical and food industries impose additional requirements when lithium hydroxide is used in their applications. The United States Pharmacopeia (USP) and Food and Drug Administration (FDA) establish specific limits for heavy metals and other contaminants, requiring validated analytical methods and regular stability testing.
Emerging certification trends include sustainability-focused standards that assess environmental impact of production processes and carbon footprint. Organizations like the Initiative for Responsible Mining Assurance (IRMA) are developing frameworks that incorporate both quality control and environmental sustainability metrics, reflecting the growing importance of ESG (Environmental, Social, and Governance) considerations in the lithium supply chain.
Laboratory accreditation represents another critical component of quality assurance. Testing facilities must typically maintain accreditation to ISO/IEC 17025 standards, demonstrating technical competence through regular proficiency testing and external audits by accreditation bodies such as A2LA in the US or UKAS in the UK.
Environmental Impact of Testing Procedures
The testing procedures for lithium hydroxide contaminants carry significant environmental implications that must be carefully considered. Traditional analytical methods often involve hazardous chemicals such as acids, organic solvents, and heavy metal reagents that pose environmental risks when improperly handled or disposed of. These chemicals can contaminate water systems, harm aquatic ecosystems, and potentially enter the food chain if released into the environment.
Atomic absorption spectroscopy and inductively coupled plasma techniques, while highly effective for detecting metal contaminants in lithium hydroxide, consume substantial energy and produce waste streams containing trace metals and argon gas. The environmental footprint extends beyond immediate laboratory waste to include the carbon emissions associated with the high energy requirements of these precision instruments.
Wet chemical methods typically generate acidic or basic waste streams that require neutralization before disposal. Without proper treatment, these wastes can alter the pH of receiving water bodies, disrupting ecological balance and potentially causing harm to sensitive aquatic organisms. Additionally, the production and transportation of reagents used in these tests contribute to upstream environmental impacts through resource extraction and manufacturing processes.
Modern green chemistry approaches are gradually transforming testing protocols to reduce environmental impact. These include miniaturization of sample sizes, replacement of toxic reagents with environmentally benign alternatives, and development of solvent-free analytical techniques. Microfluidic devices and lab-on-chip technologies represent promising advances that dramatically reduce reagent consumption and waste generation while maintaining analytical precision.
Regulatory frameworks increasingly mandate life cycle assessments of analytical procedures, encouraging laboratories to adopt more sustainable practices. This includes implementing waste recovery systems, utilizing renewable energy sources for laboratory operations, and establishing reagent recycling programs. Some facilities have successfully implemented closed-loop systems that recover and purify solvents for reuse, significantly reducing waste output.
The battery industry's rapid growth intensifies the importance of environmentally responsible testing procedures. As lithium hydroxide production scales up to meet demand for electric vehicle batteries, the cumulative environmental impact of quality control testing becomes increasingly significant. Developing standardized, environmentally sustainable testing protocols represents a critical challenge for the industry, balancing the need for rigorous contaminant detection with environmental stewardship principles.
Atomic absorption spectroscopy and inductively coupled plasma techniques, while highly effective for detecting metal contaminants in lithium hydroxide, consume substantial energy and produce waste streams containing trace metals and argon gas. The environmental footprint extends beyond immediate laboratory waste to include the carbon emissions associated with the high energy requirements of these precision instruments.
Wet chemical methods typically generate acidic or basic waste streams that require neutralization before disposal. Without proper treatment, these wastes can alter the pH of receiving water bodies, disrupting ecological balance and potentially causing harm to sensitive aquatic organisms. Additionally, the production and transportation of reagents used in these tests contribute to upstream environmental impacts through resource extraction and manufacturing processes.
Modern green chemistry approaches are gradually transforming testing protocols to reduce environmental impact. These include miniaturization of sample sizes, replacement of toxic reagents with environmentally benign alternatives, and development of solvent-free analytical techniques. Microfluidic devices and lab-on-chip technologies represent promising advances that dramatically reduce reagent consumption and waste generation while maintaining analytical precision.
Regulatory frameworks increasingly mandate life cycle assessments of analytical procedures, encouraging laboratories to adopt more sustainable practices. This includes implementing waste recovery systems, utilizing renewable energy sources for laboratory operations, and establishing reagent recycling programs. Some facilities have successfully implemented closed-loop systems that recover and purify solvents for reuse, significantly reducing waste output.
The battery industry's rapid growth intensifies the importance of environmentally responsible testing procedures. As lithium hydroxide production scales up to meet demand for electric vehicle batteries, the cumulative environmental impact of quality control testing becomes increasingly significant. Developing standardized, environmentally sustainable testing protocols represents a critical challenge for the industry, balancing the need for rigorous contaminant detection with environmental stewardship principles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!