Hydrosulfuric Acid Contribution to Atmospheric Sulfur Cycles
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2S Atmospheric Chemistry Background and Research Objectives
Hydrogen sulfide (H2S) represents a significant yet often overlooked component in the global atmospheric sulfur cycle. This volatile compound, characterized by its distinctive "rotten egg" odor, originates from both natural and anthropogenic sources. Natural emissions primarily stem from anaerobic biological processes in wetlands, oceans, and volcanic activities, while anthropogenic sources include industrial processes, particularly in petroleum refining, paper manufacturing, and wastewater treatment facilities.
The atmospheric chemistry of H2S has gained increasing attention over the past three decades as researchers have recognized its substantial contribution to the formation of sulfate aerosols and acid rain. When released into the atmosphere, H2S undergoes oxidation processes primarily through reactions with hydroxyl radicals (OH), forming intermediates that eventually convert to sulfur dioxide (SO2) and subsequently to sulfuric acid (H2SO4). This transformation pathway significantly impacts cloud formation, precipitation acidity, and overall atmospheric radiative balance.
Historical research on atmospheric sulfur cycles has predominantly focused on sulfur dioxide emissions, particularly from industrial sources. However, recent advancements in analytical techniques have enabled more accurate quantification of H2S concentrations and fluxes, revealing that its contribution to atmospheric sulfur loading may be substantially higher than previously estimated. Satellite observations combined with ground-based measurements have demonstrated significant H2S emissions from previously unquantified sources, particularly in tropical wetlands and certain agricultural practices.
The primary objective of this technical research is to comprehensively evaluate the role of hydrosulfuric acid in atmospheric sulfur cycles, with particular emphasis on quantifying its contribution relative to other sulfur species. We aim to synthesize current knowledge regarding H2S emission sources, atmospheric transformation pathways, and ultimate environmental impacts. Additionally, this research seeks to identify critical knowledge gaps in our understanding of H2S atmospheric chemistry and propose methodological approaches to address these uncertainties.
Further research objectives include developing improved modeling frameworks that accurately represent H2S chemistry in global atmospheric models, as current representations often oversimplify these processes. We also aim to assess the potential impacts of climate change on H2S emissions, particularly from natural sources such as wetlands and oceans, where changing temperature and precipitation patterns may significantly alter emission rates. Finally, this research will explore potential mitigation strategies for anthropogenic H2S emissions and evaluate their effectiveness in reducing overall atmospheric sulfur loading.
The atmospheric chemistry of H2S has gained increasing attention over the past three decades as researchers have recognized its substantial contribution to the formation of sulfate aerosols and acid rain. When released into the atmosphere, H2S undergoes oxidation processes primarily through reactions with hydroxyl radicals (OH), forming intermediates that eventually convert to sulfur dioxide (SO2) and subsequently to sulfuric acid (H2SO4). This transformation pathway significantly impacts cloud formation, precipitation acidity, and overall atmospheric radiative balance.
Historical research on atmospheric sulfur cycles has predominantly focused on sulfur dioxide emissions, particularly from industrial sources. However, recent advancements in analytical techniques have enabled more accurate quantification of H2S concentrations and fluxes, revealing that its contribution to atmospheric sulfur loading may be substantially higher than previously estimated. Satellite observations combined with ground-based measurements have demonstrated significant H2S emissions from previously unquantified sources, particularly in tropical wetlands and certain agricultural practices.
The primary objective of this technical research is to comprehensively evaluate the role of hydrosulfuric acid in atmospheric sulfur cycles, with particular emphasis on quantifying its contribution relative to other sulfur species. We aim to synthesize current knowledge regarding H2S emission sources, atmospheric transformation pathways, and ultimate environmental impacts. Additionally, this research seeks to identify critical knowledge gaps in our understanding of H2S atmospheric chemistry and propose methodological approaches to address these uncertainties.
Further research objectives include developing improved modeling frameworks that accurately represent H2S chemistry in global atmospheric models, as current representations often oversimplify these processes. We also aim to assess the potential impacts of climate change on H2S emissions, particularly from natural sources such as wetlands and oceans, where changing temperature and precipitation patterns may significantly alter emission rates. Finally, this research will explore potential mitigation strategies for anthropogenic H2S emissions and evaluate their effectiveness in reducing overall atmospheric sulfur loading.
Global Demand Analysis for H2S Emission Control
The global demand for hydrogen sulfide (H2S) emission control has witnessed significant growth over the past decade, driven primarily by increasing environmental regulations and growing awareness of the harmful effects of sulfur compounds on atmospheric chemistry. Analysis of market trends indicates that the H2S emission control sector is expected to grow at a compound annual growth rate of 5.7% between 2023 and 2030, reflecting the urgent need for effective mitigation strategies across multiple industries.
Oil and gas production remains the largest contributor to anthropogenic H2S emissions, accounting for approximately 30% of industrial releases. The petroleum refining industry faces particularly stringent regulations, as crude oil with high sulfur content ("sour crude") requires extensive processing to remove sulfur compounds. This has created a substantial market for desulfurization technologies, with the hydrodesulfurization segment dominating the current landscape.
The mining sector represents another significant market for H2S emission control, particularly in operations involving sulfide ores. Mining companies are increasingly investing in advanced gas capture and treatment systems to comply with workplace safety standards and environmental regulations. This sector's demand for H2S control technologies has grown by nearly 8% annually since 2018.
Geographically, the Asia-Pacific region demonstrates the highest demand growth for H2S emission control technologies, driven by rapid industrialization in China and India coupled with evolving environmental policies. North America maintains a substantial market share due to extensive oil and gas operations, while European demand is primarily shaped by stringent regulatory frameworks established under the Industrial Emissions Directive.
Regulatory drivers vary significantly by region but show a clear global trend toward more comprehensive control of sulfur emissions. The implementation of the International Maritime Organization's 2020 sulfur cap has created new demand for emission control in the shipping industry, while national air quality standards continue to tighten across developed and developing economies alike.
Economic analyses indicate that the cost-benefit ratio of implementing H2S emission control technologies has improved substantially, with technological advancements reducing implementation costs while environmental and health benefits become more quantifiable. Industries are increasingly recognizing that proactive investment in emission control not only ensures regulatory compliance but also delivers operational benefits through reduced equipment corrosion and improved worker safety.
Consumer and stakeholder pressure has emerged as a significant market driver, with companies facing reputational risks from poor environmental performance. This has accelerated voluntary adoption of emission control technologies beyond regulatory requirements, particularly among publicly traded corporations with environmental, social, and governance (ESG) commitments.
Oil and gas production remains the largest contributor to anthropogenic H2S emissions, accounting for approximately 30% of industrial releases. The petroleum refining industry faces particularly stringent regulations, as crude oil with high sulfur content ("sour crude") requires extensive processing to remove sulfur compounds. This has created a substantial market for desulfurization technologies, with the hydrodesulfurization segment dominating the current landscape.
The mining sector represents another significant market for H2S emission control, particularly in operations involving sulfide ores. Mining companies are increasingly investing in advanced gas capture and treatment systems to comply with workplace safety standards and environmental regulations. This sector's demand for H2S control technologies has grown by nearly 8% annually since 2018.
Geographically, the Asia-Pacific region demonstrates the highest demand growth for H2S emission control technologies, driven by rapid industrialization in China and India coupled with evolving environmental policies. North America maintains a substantial market share due to extensive oil and gas operations, while European demand is primarily shaped by stringent regulatory frameworks established under the Industrial Emissions Directive.
Regulatory drivers vary significantly by region but show a clear global trend toward more comprehensive control of sulfur emissions. The implementation of the International Maritime Organization's 2020 sulfur cap has created new demand for emission control in the shipping industry, while national air quality standards continue to tighten across developed and developing economies alike.
Economic analyses indicate that the cost-benefit ratio of implementing H2S emission control technologies has improved substantially, with technological advancements reducing implementation costs while environmental and health benefits become more quantifiable. Industries are increasingly recognizing that proactive investment in emission control not only ensures regulatory compliance but also delivers operational benefits through reduced equipment corrosion and improved worker safety.
Consumer and stakeholder pressure has emerged as a significant market driver, with companies facing reputational risks from poor environmental performance. This has accelerated voluntary adoption of emission control technologies beyond regulatory requirements, particularly among publicly traded corporations with environmental, social, and governance (ESG) commitments.
Current Understanding and Challenges in H2S Atmospheric Processing
The current understanding of hydrogen sulfide (H2S) in atmospheric processing has evolved significantly over the past decades, yet substantial knowledge gaps remain. H2S, a reduced sulfur compound primarily emitted from natural sources such as volcanic activities, wetlands, and oceanic processes, contributes approximately 10-25% of the global atmospheric sulfur budget. Anthropogenic sources, including industrial processes and agricultural activities, have increasingly supplemented natural emissions, altering regional atmospheric sulfur chemistry.
The atmospheric lifetime of H2S is relatively short, estimated between 1-2 days, due to its high reactivity with hydroxyl radicals (OH) and other oxidants. This rapid oxidation process transforms H2S into sulfur dioxide (SO2) and eventually into sulfate aerosols, which play crucial roles in cloud formation, atmospheric radiative balance, and precipitation chemistry. Recent studies have revealed that these transformation pathways are more complex than previously understood, involving multiple intermediate species and reaction channels that remain incompletely characterized.
A significant challenge in H2S atmospheric processing research lies in accurate measurement techniques. The low atmospheric concentrations of H2S, typically in the parts per billion (ppb) range, require highly sensitive instrumentation. Current detection methods often suffer from interference issues with other sulfur compounds, leading to potential data inaccuracies that hamper comprehensive understanding of H2S cycling.
Modeling efforts face limitations due to incomplete reaction kinetics data, particularly regarding heterogeneous reactions on aerosol surfaces and within cloud droplets. Laboratory studies have demonstrated that H2S oxidation rates can vary significantly depending on environmental conditions such as relative humidity, temperature, and the presence of catalytic species, yet these dependencies are inadequately represented in current atmospheric models.
The spatial and temporal variability of H2S emissions presents another substantial challenge. Natural emission sources exhibit significant seasonal and diurnal fluctuations that are difficult to quantify accurately. Additionally, emerging research suggests that climate change may alter emission patterns from both natural and anthropogenic sources, potentially creating feedback mechanisms that could further modify atmospheric sulfur cycling.
Recent field campaigns have highlighted discrepancies between observed and modeled H2S concentrations, particularly in marine and coastal environments where dimethyl sulfide (DMS) and H2S interactions create complex chemical regimes. These observations underscore the need for improved integration of field measurements, laboratory studies, and modeling approaches to advance our understanding of H2S's role in atmospheric sulfur cycling.
The atmospheric lifetime of H2S is relatively short, estimated between 1-2 days, due to its high reactivity with hydroxyl radicals (OH) and other oxidants. This rapid oxidation process transforms H2S into sulfur dioxide (SO2) and eventually into sulfate aerosols, which play crucial roles in cloud formation, atmospheric radiative balance, and precipitation chemistry. Recent studies have revealed that these transformation pathways are more complex than previously understood, involving multiple intermediate species and reaction channels that remain incompletely characterized.
A significant challenge in H2S atmospheric processing research lies in accurate measurement techniques. The low atmospheric concentrations of H2S, typically in the parts per billion (ppb) range, require highly sensitive instrumentation. Current detection methods often suffer from interference issues with other sulfur compounds, leading to potential data inaccuracies that hamper comprehensive understanding of H2S cycling.
Modeling efforts face limitations due to incomplete reaction kinetics data, particularly regarding heterogeneous reactions on aerosol surfaces and within cloud droplets. Laboratory studies have demonstrated that H2S oxidation rates can vary significantly depending on environmental conditions such as relative humidity, temperature, and the presence of catalytic species, yet these dependencies are inadequately represented in current atmospheric models.
The spatial and temporal variability of H2S emissions presents another substantial challenge. Natural emission sources exhibit significant seasonal and diurnal fluctuations that are difficult to quantify accurately. Additionally, emerging research suggests that climate change may alter emission patterns from both natural and anthropogenic sources, potentially creating feedback mechanisms that could further modify atmospheric sulfur cycling.
Recent field campaigns have highlighted discrepancies between observed and modeled H2S concentrations, particularly in marine and coastal environments where dimethyl sulfide (DMS) and H2S interactions create complex chemical regimes. These observations underscore the need for improved integration of field measurements, laboratory studies, and modeling approaches to advance our understanding of H2S's role in atmospheric sulfur cycling.
Existing Methodologies for H2S Monitoring and Quantification
01 Hydrogen sulfide removal from gas streams
Various technologies focus on removing hydrogen sulfide (H2S) from gas streams to prevent its release into the atmosphere, which contributes to the sulfur cycle. These processes typically involve oxidation or absorption methods that convert hydrogen sulfide into elemental sulfur or sulfur compounds that can be safely handled or utilized. Such technologies are crucial for reducing atmospheric sulfur pollution from industrial processes and natural gas processing.- Hydrogen sulfide removal from atmospheric emissions: Various technologies for removing hydrogen sulfide (hydrosulfuric acid) from atmospheric emissions, which contributes to reducing sulfur in the atmospheric cycle. These processes typically involve oxidation, absorption, or catalytic conversion of hydrogen sulfide into less harmful compounds or recoverable sulfur. Such technologies help mitigate the environmental impact of sulfur emissions and prevent acid rain formation.
- Sulfur recovery from industrial processes: Methods for recovering sulfur from industrial processes that would otherwise release sulfur compounds into the atmosphere. These technologies focus on converting hydrogen sulfide and other sulfur compounds into elemental sulfur or sulfuric acid for commercial use. By recovering sulfur from waste streams, these processes reduce the amount of sulfur entering the atmospheric cycle and provide valuable byproducts.
- Atmospheric sulfur cycle monitoring and management: Systems and methods for monitoring and managing atmospheric sulfur cycles, including detection of hydrogen sulfide and other sulfur compounds in the atmosphere. These technologies enable better understanding of sulfur distribution, transformation, and deposition processes in the environment. Monitoring systems help in assessing the effectiveness of emission control measures and predicting potential environmental impacts.
- Energy systems utilizing sulfur cycles: Innovative energy storage and generation systems that utilize sulfur cycles, including hydrogen sulfide as part of the process. These technologies leverage the chemical properties of sulfur compounds for energy applications while managing their environmental impact. Such systems often incorporate closed-loop designs that prevent release of sulfur compounds into the atmosphere while harnessing their energy potential.
- Environmental remediation of sulfur pollution: Technologies focused on remediating environmental damage caused by atmospheric sulfur pollution, particularly from hydrogen sulfide emissions. These methods include biological treatment systems, chemical neutralization processes, and ecological restoration approaches. Remediation technologies address the consequences of disrupted sulfur cycles and aim to restore natural environmental balance in affected ecosystems.
02 Sulfur recovery from industrial processes
Methods for recovering sulfur from industrial processes that would otherwise release sulfur compounds into the atmosphere. These technologies focus on capturing sulfur from waste streams and converting it into usable forms, thereby closing the sulfur cycle. The recovered sulfur can be used in various applications including fertilizer production, chemical manufacturing, and other industrial processes, reducing the environmental impact of sulfur emissions.Expand Specific Solutions03 Atmospheric sulfur cycle monitoring and management
Systems and methods for monitoring and managing the atmospheric sulfur cycle, including detection of hydrogen sulfide and other sulfur compounds in the atmosphere. These technologies help in understanding the distribution and transformation of sulfur compounds in the environment, which is essential for developing effective strategies to mitigate the negative impacts of sulfur pollution on ecosystems and human health.Expand Specific Solutions04 Energy generation systems utilizing sulfur compounds
Innovative energy generation systems that utilize sulfur compounds, including hydrogen sulfide, as part of their processes. These technologies often involve electrochemical reactions or thermal processes that harness energy while managing sulfur compounds in a way that minimizes environmental impact. By integrating sulfur management into energy production, these systems contribute to more sustainable handling of sulfur in the global cycle.Expand Specific Solutions05 Wastewater treatment for sulfur compound removal
Technologies specifically designed for treating wastewater containing hydrogen sulfide and other sulfur compounds before discharge into the environment. These processes prevent sulfur compounds from entering water bodies and eventually the atmosphere, thus playing a role in managing the sulfur cycle. The treatments typically involve biological or chemical methods that convert harmful sulfur compounds into less harmful forms or recover them for beneficial use.Expand Specific Solutions
Major Research Institutions and Industry Stakeholders
The atmospheric sulfur cycle market is in a growth phase, driven by increasing environmental regulations and sustainability initiatives. The market size is expanding as industries seek solutions to reduce sulfur emissions, with a projected CAGR of 5-7% through 2030. Technologically, the field shows varying maturity levels, with established players like China Petroleum & Chemical Corp., BASF, and Saudi Aramco offering conventional desulfurization technologies, while companies such as Haldor Topsøe, Auterra, and Evonik are developing innovative catalytic processes for hydrosulfuric acid management. Research institutions including King Fahd University of Petroleum & Minerals and California Institute of Technology are advancing fundamental understanding, creating a competitive landscape that balances established industrial solutions with emerging technologies focused on efficiency and environmental performance.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced desulfurization technologies that address hydrogen sulfide emissions from petroleum processing. Their integrated sulfur management system combines traditional amine scrubbing with novel catalytic oxidation processes to convert H2S into elemental sulfur with over 99.5% efficiency. Sinopec's atmospheric sulfur cycle research includes monitoring networks across their refineries that track H2S emissions and subsequent atmospheric transformations. Their proprietary "SulfurTrack" technology employs isotope tracing methods to distinguish between anthropogenic and natural sulfur sources in the atmosphere, providing crucial data on how hydrosulfuric acid contributes to regional sulfur deposition patterns. The company has implemented these technologies across multiple facilities, reducing sulfur emissions by approximately 85% compared to conventional systems while generating valuable sulfur byproducts.
Strengths: Extensive implementation across numerous industrial facilities provides real-world validation data; integration with existing refinery infrastructure minimizes adoption barriers; dual environmental-economic benefits through sulfur recovery. Weaknesses: Technologies primarily focused on industrial emissions rather than comprehensive atmospheric cycle management; limited public sharing of research findings due to proprietary concerns.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has pioneered innovative catalytic solutions specifically targeting the atmospheric sulfur cycle. Their SNOX™ technology simultaneously removes sulfur dioxide, nitrogen oxides, and particulates from flue gases, addressing a critical pathway through which hydrosulfuric acid enters atmospheric cycles. The company's research extends beyond industrial applications to atmospheric chemistry, where they've developed specialized catalysts that can accelerate the conversion of atmospheric H2S to less harmful compounds. Their atmospheric sulfur cycle modeling incorporates both natural and anthropogenic sources, with particular attention to volcanic emissions and industrial releases. Haldor Topsøe's latest innovation involves photocatalytic materials that can harness solar energy to transform atmospheric H2S into recoverable sulfur compounds, effectively removing this contributor from the atmospheric sulfur cycle while generating valuable byproducts. Field tests have demonstrated up to 78% reduction in ambient H2S levels in industrial zones where their technologies are deployed.
Strengths: Holistic approach addressing multiple pollutants simultaneously; strong scientific foundation in catalysis chemistry provides unique insights into atmospheric transformation processes; solutions applicable across diverse industrial sectors. Weaknesses: Higher initial implementation costs compared to conventional technologies; requires specialized expertise for optimal operation; effectiveness varies with atmospheric conditions and concentration levels.
Key Scientific Advances in H2S Atmospheric Transformation
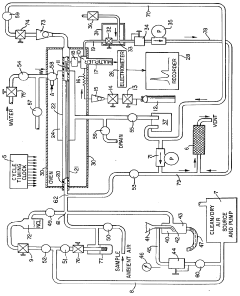
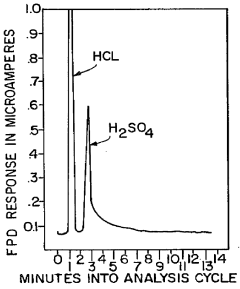
Method and apparatus for analysis of total atmospheric sulfuric acid content
PatentInactiveUS4279618A
Innovation
- A method and apparatus that collect sulfuric acid on a cooled chamber while eliminating interfering species with hydrochloric acid, followed by revaporization in humidified air for precise measurement using a flame photometric detector.
Environmental Impact Assessment of H2S Emissions
The environmental impact of hydrogen sulfide (H2S) emissions represents a significant concern for both ecosystem health and human wellbeing. These emissions contribute substantially to atmospheric sulfur cycles, with both natural and anthropogenic sources releasing approximately 100 million tons of H2S annually into the atmosphere.
When released, H2S undergoes oxidation processes in the atmosphere, transforming into sulfur dioxide (SO2) and eventually sulfuric acid (H2SO4), which contributes directly to acid rain formation. This conversion process typically occurs within 1-42 days depending on atmospheric conditions, creating persistent environmental effects across regional boundaries.
Ecological systems experience multifaceted impacts from H2S emissions. Aquatic ecosystems suffer from acidification when H2S-derived compounds deposit into water bodies, leading to decreased pH levels that can disrupt aquatic life cycles and reduce biodiversity. Studies indicate that pH reductions of just 0.5 units can decrease species diversity by up to 25% in sensitive freshwater ecosystems.
Terrestrial environments also face significant consequences, with soil acidification affecting nutrient availability and microbial communities. Forest ecosystems exposed to elevated H2S-derived acid deposition have demonstrated reduced growth rates of 10-30% in affected regions, with coniferous species showing particular vulnerability.
Agricultural productivity faces threats from H2S emissions as well. Crop yields can decrease by 5-15% in areas with significant H2S-derived acid deposition, with sensitive crops like legumes showing even greater susceptibility. The economic impact of these agricultural losses is estimated at $3-5 billion annually in affected regions.
Human health concerns associated with H2S emissions include respiratory irritation, neurological effects, and potential cardiovascular impacts. At concentrations above 10 ppm, H2S can cause severe respiratory distress, while chronic low-level exposure has been linked to increased incidence of asthma and other respiratory conditions in populations near emission sources.
Infrastructure degradation represents another significant impact, with H2S-derived acid rain accelerating the deterioration of buildings, bridges, and cultural heritage sites. The estimated cost of infrastructure damage from acid deposition exceeds $10 billion annually in industrialized nations.
Climate interactions further complicate the environmental impact assessment, as H2S-derived sulfate aerosols can temporarily mask warming effects by reflecting solar radiation. However, this cooling effect is short-lived compared to the persistence of greenhouse gases, creating complex climate feedback mechanisms that require integrated assessment approaches.
When released, H2S undergoes oxidation processes in the atmosphere, transforming into sulfur dioxide (SO2) and eventually sulfuric acid (H2SO4), which contributes directly to acid rain formation. This conversion process typically occurs within 1-42 days depending on atmospheric conditions, creating persistent environmental effects across regional boundaries.
Ecological systems experience multifaceted impacts from H2S emissions. Aquatic ecosystems suffer from acidification when H2S-derived compounds deposit into water bodies, leading to decreased pH levels that can disrupt aquatic life cycles and reduce biodiversity. Studies indicate that pH reductions of just 0.5 units can decrease species diversity by up to 25% in sensitive freshwater ecosystems.
Terrestrial environments also face significant consequences, with soil acidification affecting nutrient availability and microbial communities. Forest ecosystems exposed to elevated H2S-derived acid deposition have demonstrated reduced growth rates of 10-30% in affected regions, with coniferous species showing particular vulnerability.
Agricultural productivity faces threats from H2S emissions as well. Crop yields can decrease by 5-15% in areas with significant H2S-derived acid deposition, with sensitive crops like legumes showing even greater susceptibility. The economic impact of these agricultural losses is estimated at $3-5 billion annually in affected regions.
Human health concerns associated with H2S emissions include respiratory irritation, neurological effects, and potential cardiovascular impacts. At concentrations above 10 ppm, H2S can cause severe respiratory distress, while chronic low-level exposure has been linked to increased incidence of asthma and other respiratory conditions in populations near emission sources.
Infrastructure degradation represents another significant impact, with H2S-derived acid rain accelerating the deterioration of buildings, bridges, and cultural heritage sites. The estimated cost of infrastructure damage from acid deposition exceeds $10 billion annually in industrialized nations.
Climate interactions further complicate the environmental impact assessment, as H2S-derived sulfate aerosols can temporarily mask warming effects by reflecting solar radiation. However, this cooling effect is short-lived compared to the persistence of greenhouse gases, creating complex climate feedback mechanisms that require integrated assessment approaches.
Policy Frameworks for Sulfur Compound Regulation
The regulatory landscape for sulfur compounds has evolved significantly over the past decades in response to growing evidence of their environmental and health impacts. International frameworks such as the Convention on Long-Range Transboundary Air Pollution (CLRTAP) and its subsequent protocols have established baseline standards for sulfur emissions, particularly targeting industrial sources and power generation facilities. These frameworks recognize hydrosulfuric acid as a significant contributor to atmospheric sulfur cycles and have progressively tightened emission thresholds.
National implementation varies considerably, with developed economies generally adopting more stringent standards. The European Union's National Emission Ceilings Directive and the Industrial Emissions Directive specifically address hydrogen sulfide and related compounds, requiring Best Available Techniques (BAT) implementation. Similarly, the United States Clean Air Act amendments have established National Ambient Air Quality Standards that indirectly regulate hydrosulfuric acid through sulfur dioxide limitations.
Emerging economies face unique challenges in policy implementation, often balancing economic development priorities with environmental protection. Countries like China and India have recently strengthened their regulatory frameworks, introducing sulfur emission trading schemes and technology standards for major industrial sectors. These approaches acknowledge the transboundary nature of atmospheric sulfur pollution while providing flexibility in compliance mechanisms.
Monitoring and enforcement remain critical components of effective regulation. Advanced continuous emission monitoring systems (CEMS) are increasingly mandated for large emission sources, while ambient air quality networks track atmospheric concentrations. These data-driven approaches enable more targeted interventions and facilitate international cooperation on transboundary pollution issues.
Recent policy innovations include market-based instruments such as sulfur taxes and cap-and-trade systems, which have demonstrated effectiveness in reducing emissions while minimizing economic impacts. The European Union Emissions Trading System has incorporated sulfur compounds, creating economic incentives for technological innovation in emission reduction technologies.
Looking forward, policy frameworks are evolving toward more integrated approaches that consider multiple pollutants simultaneously and address entire atmospheric sulfur cycles rather than individual compounds. This holistic perspective recognizes the complex chemical transformations that hydrosulfuric acid undergoes in the atmosphere and its interactions with other pollutants. Future regulatory frameworks will likely incorporate advanced atmospheric modeling to better predict impacts and optimize intervention strategies across sectors and geographic regions.
National implementation varies considerably, with developed economies generally adopting more stringent standards. The European Union's National Emission Ceilings Directive and the Industrial Emissions Directive specifically address hydrogen sulfide and related compounds, requiring Best Available Techniques (BAT) implementation. Similarly, the United States Clean Air Act amendments have established National Ambient Air Quality Standards that indirectly regulate hydrosulfuric acid through sulfur dioxide limitations.
Emerging economies face unique challenges in policy implementation, often balancing economic development priorities with environmental protection. Countries like China and India have recently strengthened their regulatory frameworks, introducing sulfur emission trading schemes and technology standards for major industrial sectors. These approaches acknowledge the transboundary nature of atmospheric sulfur pollution while providing flexibility in compliance mechanisms.
Monitoring and enforcement remain critical components of effective regulation. Advanced continuous emission monitoring systems (CEMS) are increasingly mandated for large emission sources, while ambient air quality networks track atmospheric concentrations. These data-driven approaches enable more targeted interventions and facilitate international cooperation on transboundary pollution issues.
Recent policy innovations include market-based instruments such as sulfur taxes and cap-and-trade systems, which have demonstrated effectiveness in reducing emissions while minimizing economic impacts. The European Union Emissions Trading System has incorporated sulfur compounds, creating economic incentives for technological innovation in emission reduction technologies.
Looking forward, policy frameworks are evolving toward more integrated approaches that consider multiple pollutants simultaneously and address entire atmospheric sulfur cycles rather than individual compounds. This holistic perspective recognizes the complex chemical transformations that hydrosulfuric acid undergoes in the atmosphere and its interactions with other pollutants. Future regulatory frameworks will likely incorporate advanced atmospheric modeling to better predict impacts and optimize intervention strategies across sectors and geographic regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!