Innovations in Sodium Percarbonate Stabilization Techniques
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Evolution and Objectives
Sodium percarbonate, a versatile compound widely used in cleaning and bleaching applications, has undergone significant evolution since its discovery in the early 20th century. Initially developed as an alternative to sodium perborate, sodium percarbonate has gained prominence due to its environmental friendliness and superior performance in various applications.
The evolution of sodium percarbonate can be traced through several key milestones. In the 1960s, the first commercial production of sodium percarbonate began, marking a significant shift in the cleaning industry. The 1980s saw increased research into stabilization techniques, addressing the compound's inherent instability in storage and application. By the 1990s, sodium percarbonate had become a staple in laundry detergents, replacing less eco-friendly alternatives.
Recent years have witnessed a surge in research focused on enhancing the stability and efficacy of sodium percarbonate. This renewed interest is driven by the growing demand for sustainable and efficient cleaning solutions across various industries. The primary objective in this field is to develop innovative stabilization techniques that can overcome the compound's sensitivity to moisture, temperature, and certain metal ions.
Current research objectives in sodium percarbonate stabilization encompass several key areas. Firstly, there is a push to improve the compound's shelf life, ensuring it remains effective over extended periods without significant degradation. Secondly, researchers are exploring ways to enhance its stability in aqueous solutions, broadening its applicability in liquid formulations. Another critical objective is to develop stabilization methods that are cost-effective and scalable for industrial production.
The environmental impact of stabilization techniques is also a paramount concern. Researchers are striving to develop eco-friendly stabilizers that do not compromise the green credentials of sodium percarbonate. This aligns with the broader industry trend towards sustainable chemistry and reduced environmental footprint.
Additionally, there is a focus on tailoring sodium percarbonate formulations for specific applications. This includes developing stabilized versions that perform optimally in different pH ranges, temperatures, and in the presence of various chemical compounds commonly found in cleaning and bleaching formulations.
As we look to the future, the objectives for sodium percarbonate stabilization are likely to expand. Emerging areas of interest include the development of smart release mechanisms, allowing for controlled and targeted release of the active oxygen. There is also growing interest in combining sodium percarbonate with other active ingredients to create multi-functional cleaning agents, necessitating new approaches to stabilization that can accommodate these complex formulations.
The evolution of sodium percarbonate can be traced through several key milestones. In the 1960s, the first commercial production of sodium percarbonate began, marking a significant shift in the cleaning industry. The 1980s saw increased research into stabilization techniques, addressing the compound's inherent instability in storage and application. By the 1990s, sodium percarbonate had become a staple in laundry detergents, replacing less eco-friendly alternatives.
Recent years have witnessed a surge in research focused on enhancing the stability and efficacy of sodium percarbonate. This renewed interest is driven by the growing demand for sustainable and efficient cleaning solutions across various industries. The primary objective in this field is to develop innovative stabilization techniques that can overcome the compound's sensitivity to moisture, temperature, and certain metal ions.
Current research objectives in sodium percarbonate stabilization encompass several key areas. Firstly, there is a push to improve the compound's shelf life, ensuring it remains effective over extended periods without significant degradation. Secondly, researchers are exploring ways to enhance its stability in aqueous solutions, broadening its applicability in liquid formulations. Another critical objective is to develop stabilization methods that are cost-effective and scalable for industrial production.
The environmental impact of stabilization techniques is also a paramount concern. Researchers are striving to develop eco-friendly stabilizers that do not compromise the green credentials of sodium percarbonate. This aligns with the broader industry trend towards sustainable chemistry and reduced environmental footprint.
Additionally, there is a focus on tailoring sodium percarbonate formulations for specific applications. This includes developing stabilized versions that perform optimally in different pH ranges, temperatures, and in the presence of various chemical compounds commonly found in cleaning and bleaching formulations.
As we look to the future, the objectives for sodium percarbonate stabilization are likely to expand. Emerging areas of interest include the development of smart release mechanisms, allowing for controlled and targeted release of the active oxygen. There is also growing interest in combining sodium percarbonate with other active ingredients to create multi-functional cleaning agents, necessitating new approaches to stabilization that can accommodate these complex formulations.
Market Analysis for Stabilized Sodium Percarbonate
The global market for stabilized sodium percarbonate has been experiencing steady growth, driven by increasing demand for eco-friendly and efficient cleaning products across various industries. This compound, known for its powerful oxidizing properties and environmental sustainability, has found widespread applications in laundry detergents, household cleaners, and industrial cleaning processes.
In recent years, the market has witnessed a significant shift towards green and sustainable cleaning solutions, particularly in developed regions such as North America and Europe. This trend has been a major factor propelling the demand for stabilized sodium percarbonate, as it offers an effective alternative to chlorine-based bleaching agents. The compound's ability to release active oxygen when dissolved in water makes it an attractive option for consumers seeking powerful yet environmentally responsible cleaning products.
The laundry detergent sector remains the largest consumer of stabilized sodium percarbonate, accounting for a substantial portion of the market share. The growing awareness of hygiene and cleanliness, especially in the wake of global health concerns, has further boosted the demand for high-performance laundry products. This has led to increased incorporation of stabilized sodium percarbonate in both powder and liquid detergent formulations.
Industrial cleaning applications have also shown promising growth potential for stabilized sodium percarbonate. Industries such as food processing, healthcare, and hospitality are increasingly adopting this compound for its effectiveness in removing tough stains and disinfecting surfaces. The compound's ability to work at lower temperatures compared to traditional bleaching agents has made it particularly attractive for energy-efficient cleaning processes.
Geographically, Asia-Pacific has emerged as a rapidly growing market for stabilized sodium percarbonate. The region's expanding middle-class population, coupled with rising disposable incomes and changing consumer preferences, has led to increased demand for premium cleaning products. Countries like China and India are expected to be key growth drivers in the coming years, presenting significant opportunities for market players.
However, the market faces challenges in terms of raw material price fluctuations and competition from alternative bleaching agents. The production of sodium percarbonate relies heavily on the availability and pricing of key raw materials such as hydrogen peroxide and soda ash. Volatility in these input costs can impact the overall market dynamics and profitability of manufacturers.
Looking ahead, the market for stabilized sodium percarbonate is projected to continue its growth trajectory. Factors such as increasing urbanization, growing awareness of sustainable cleaning solutions, and stringent regulations on harmful chemicals in consumer products are expected to drive market expansion. Innovations in stabilization techniques and product formulations will play a crucial role in addressing current limitations and unlocking new application areas for this versatile compound.
In recent years, the market has witnessed a significant shift towards green and sustainable cleaning solutions, particularly in developed regions such as North America and Europe. This trend has been a major factor propelling the demand for stabilized sodium percarbonate, as it offers an effective alternative to chlorine-based bleaching agents. The compound's ability to release active oxygen when dissolved in water makes it an attractive option for consumers seeking powerful yet environmentally responsible cleaning products.
The laundry detergent sector remains the largest consumer of stabilized sodium percarbonate, accounting for a substantial portion of the market share. The growing awareness of hygiene and cleanliness, especially in the wake of global health concerns, has further boosted the demand for high-performance laundry products. This has led to increased incorporation of stabilized sodium percarbonate in both powder and liquid detergent formulations.
Industrial cleaning applications have also shown promising growth potential for stabilized sodium percarbonate. Industries such as food processing, healthcare, and hospitality are increasingly adopting this compound for its effectiveness in removing tough stains and disinfecting surfaces. The compound's ability to work at lower temperatures compared to traditional bleaching agents has made it particularly attractive for energy-efficient cleaning processes.
Geographically, Asia-Pacific has emerged as a rapidly growing market for stabilized sodium percarbonate. The region's expanding middle-class population, coupled with rising disposable incomes and changing consumer preferences, has led to increased demand for premium cleaning products. Countries like China and India are expected to be key growth drivers in the coming years, presenting significant opportunities for market players.
However, the market faces challenges in terms of raw material price fluctuations and competition from alternative bleaching agents. The production of sodium percarbonate relies heavily on the availability and pricing of key raw materials such as hydrogen peroxide and soda ash. Volatility in these input costs can impact the overall market dynamics and profitability of manufacturers.
Looking ahead, the market for stabilized sodium percarbonate is projected to continue its growth trajectory. Factors such as increasing urbanization, growing awareness of sustainable cleaning solutions, and stringent regulations on harmful chemicals in consumer products are expected to drive market expansion. Innovations in stabilization techniques and product formulations will play a crucial role in addressing current limitations and unlocking new application areas for this versatile compound.
Current Challenges in Sodium Percarbonate Stability
Sodium percarbonate, a widely used bleaching and cleaning agent, faces several stability challenges that hinder its effectiveness and shelf life. One of the primary issues is its susceptibility to moisture-induced decomposition. When exposed to humidity, sodium percarbonate can prematurely release hydrogen peroxide, leading to a significant loss of its active oxygen content and reducing its cleaning power.
Temperature fluctuations pose another major challenge to sodium percarbonate stability. High temperatures accelerate the decomposition process, while low temperatures can cause the compound to absorb moisture more readily, both scenarios resulting in reduced efficacy. This sensitivity to temperature variations complicates storage and transportation requirements, particularly in regions with diverse climates.
The presence of metal ions, especially transition metals like iron and copper, catalyzes the decomposition of sodium percarbonate. These metal impurities can originate from various sources, including manufacturing equipment, packaging materials, or even trace amounts in the water used during production. The catalytic effect of these ions significantly reduces the stability and shelf life of sodium percarbonate-based products.
Another challenge lies in the formulation of sodium percarbonate with other cleaning agents and additives. Certain compounds, such as enzymes or surfactants, can interact negatively with sodium percarbonate, leading to decreased stability or altered performance characteristics. Balancing the effectiveness of the overall cleaning formulation while maintaining sodium percarbonate stability remains a complex task for product developers.
The physical form of sodium percarbonate also impacts its stability. Fine particles have a larger surface area exposed to environmental factors, making them more prone to degradation. Conversely, larger granules may offer improved stability but can present dissolution issues in certain applications. Achieving the optimal particle size distribution that balances stability and functionality is an ongoing challenge in sodium percarbonate production.
Packaging plays a crucial role in sodium percarbonate stability, yet it presents its own set of challenges. While moisture-resistant packaging is essential, it must also be cost-effective and environmentally friendly. Additionally, the packaging must withstand the potential pressure build-up from oxygen release during storage, especially in warmer climates or during extended periods.
Addressing these stability challenges is crucial for improving the performance and longevity of sodium percarbonate-based products. Innovations in stabilization techniques are needed to overcome these hurdles and enhance the overall reliability and effectiveness of this important cleaning agent across various applications and environmental conditions.
Temperature fluctuations pose another major challenge to sodium percarbonate stability. High temperatures accelerate the decomposition process, while low temperatures can cause the compound to absorb moisture more readily, both scenarios resulting in reduced efficacy. This sensitivity to temperature variations complicates storage and transportation requirements, particularly in regions with diverse climates.
The presence of metal ions, especially transition metals like iron and copper, catalyzes the decomposition of sodium percarbonate. These metal impurities can originate from various sources, including manufacturing equipment, packaging materials, or even trace amounts in the water used during production. The catalytic effect of these ions significantly reduces the stability and shelf life of sodium percarbonate-based products.
Another challenge lies in the formulation of sodium percarbonate with other cleaning agents and additives. Certain compounds, such as enzymes or surfactants, can interact negatively with sodium percarbonate, leading to decreased stability or altered performance characteristics. Balancing the effectiveness of the overall cleaning formulation while maintaining sodium percarbonate stability remains a complex task for product developers.
The physical form of sodium percarbonate also impacts its stability. Fine particles have a larger surface area exposed to environmental factors, making them more prone to degradation. Conversely, larger granules may offer improved stability but can present dissolution issues in certain applications. Achieving the optimal particle size distribution that balances stability and functionality is an ongoing challenge in sodium percarbonate production.
Packaging plays a crucial role in sodium percarbonate stability, yet it presents its own set of challenges. While moisture-resistant packaging is essential, it must also be cost-effective and environmentally friendly. Additionally, the packaging must withstand the potential pressure build-up from oxygen release during storage, especially in warmer climates or during extended periods.
Addressing these stability challenges is crucial for improving the performance and longevity of sodium percarbonate-based products. Innovations in stabilization techniques are needed to overcome these hurdles and enhance the overall reliability and effectiveness of this important cleaning agent across various applications and environmental conditions.
Existing Stabilization Methods for Sodium Percarbonate
01 Coating with inorganic compounds
Sodium percarbonate can be stabilized by coating it with inorganic compounds such as silicates, borates, or phosphates. This coating forms a protective layer around the sodium percarbonate particles, preventing moisture absorption and improving storage stability.- Coating with inorganic compounds: Stabilization of sodium percarbonate can be achieved by coating the particles with inorganic compounds. This method creates a protective layer around the sodium percarbonate particles, preventing moisture absorption and decomposition. Common inorganic coating materials include silicates, borates, and carbonates.
- Addition of organic stabilizers: Organic stabilizers can be incorporated into sodium percarbonate formulations to enhance stability. These stabilizers, such as chelating agents or antioxidants, help prevent the decomposition of sodium percarbonate by scavenging free radicals or binding metal ions that catalyze decomposition reactions.
- Moisture control and packaging: Controlling moisture content and using appropriate packaging materials are crucial for sodium percarbonate stabilization. Reducing moisture exposure during storage and transportation helps maintain the product's stability. Specialized packaging materials and designs can be employed to create a barrier against humidity.
- Particle size and shape optimization: Optimizing the particle size and shape of sodium percarbonate can improve its stability. Larger particles or specific shapes may reduce surface area exposure, thereby decreasing the rate of decomposition. Additionally, uniform particle size distribution can enhance overall product stability.
- Temperature and pH control: Maintaining appropriate temperature and pH conditions during production, storage, and use of sodium percarbonate is essential for its stabilization. Controlling these parameters helps minimize decomposition reactions and extends the product's shelf life. Specific temperature ranges and pH levels can be optimized for maximum stability.
02 Addition of organic stabilizers
Organic stabilizers, such as carboxymethyl cellulose, polyethylene glycol, or other polymers, can be added to sodium percarbonate formulations. These stabilizers help to reduce moisture absorption and improve the overall stability of the product during storage and handling.Expand Specific Solutions03 Particle size control
Controlling the particle size of sodium percarbonate can significantly impact its stability. Larger particles generally exhibit better stability due to reduced surface area and lower moisture absorption. Techniques such as granulation or agglomeration can be used to achieve optimal particle size distribution.Expand Specific Solutions04 Moisture-resistant packaging
Utilizing moisture-resistant packaging materials and techniques can help maintain the stability of sodium percarbonate during storage and transportation. This may include the use of barrier films, desiccants, or hermetically sealed containers to minimize exposure to moisture and atmospheric humidity.Expand Specific Solutions05 Temperature and humidity control
Maintaining appropriate temperature and humidity conditions during storage and handling is crucial for sodium percarbonate stability. Implementing controlled storage environments with low humidity and moderate temperatures can significantly extend the shelf life and maintain the efficacy of the product.Expand Specific Solutions
Key Industry Players in Percarbonate Production
The sodium percarbonate stabilization techniques market is in a growth phase, driven by increasing demand for eco-friendly cleaning products. The global market size is projected to expand significantly in the coming years, with key players like Solvay SA, Evonik Operations GmbH, and Kemira Oyj leading innovation efforts. These companies are investing heavily in R&D to improve product stability and performance. The technology is reaching maturity, with advanced formulations being developed by industry leaders such as Henkel AG & Co. KGaA and Unilever. Emerging players like Zhejiang Jinke Daily Chemical Co. Ltd. are also making strides in developing novel stabilization methods, intensifying competition in this rapidly evolving sector.
Solvay SA
Technical Solution: Solvay has developed advanced stabilization techniques for sodium percarbonate, focusing on improving its stability and shelf life. Their approach involves a multi-layer coating process, utilizing silica-based materials and organic compounds. This method creates a protective barrier around the sodium percarbonate particles, significantly reducing moisture absorption and decomposition[1]. The company has also implemented a controlled release mechanism, allowing for gradual activation of the percarbonate when exposed to water, enhancing its effectiveness in various applications[3]. Additionally, Solvay has invested in particle size optimization, which contributes to better flowability and reduced caking during storage[5].
Strengths: Enhanced stability, longer shelf life, and improved performance in various applications. Weaknesses: Potentially higher production costs and complexity in manufacturing process.
Degussa AG
Technical Solution: Degussa AG, now part of Evonik Industries, has pioneered innovative stabilization techniques for sodium percarbonate. Their approach focuses on surface modification and encapsulation technologies. The company has developed a proprietary process that involves coating sodium percarbonate particles with a thin layer of inorganic salts, such as sodium silicate or sodium carbonate[2]. This coating acts as a barrier against moisture and catalytic impurities, significantly improving the stability of the product. Furthermore, Degussa has implemented a controlled crystallization process, which results in more uniform particle size distribution and enhanced bulk density[4]. This innovation not only improves the product's stability but also its handling characteristics.
Strengths: Improved product stability, enhanced handling properties, and increased efficiency in applications. Weaknesses: Potential limitations in extreme environmental conditions and higher production costs.
Breakthrough Patents in Percarbonate Stabilization
Method for the preparation of sodium percarbonate granules having enhanced stability
PatentInactiveEP1227063B1
Innovation
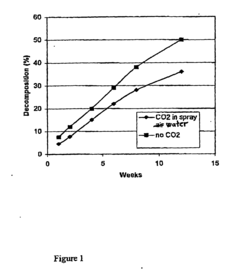
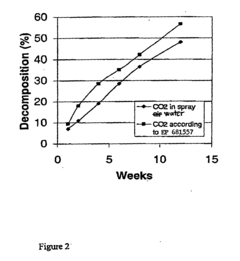
- A method involving the formation of a dense thin film of sodium bicarbonate on the surface of sodium percarbonate granules using carbon dioxide dissolved in water, which is sprayed onto the granules in a fluidized bed reactor, followed by drying, to enhance stability, with optional additional coating layers.
Stabilized percarbonate (II)
PatentWO1993020007A1
Innovation
- Coating sodium percarbonate with a liquid medium predominantly consisting of long-chain aliphatic carboxylic acids, which are liquid at room temperature, and subsequent powdering with a hydrophilic solid to enhance stability and pourability, while maintaining rapid dissolution in water.
Environmental Impact of Stabilization Processes
The environmental impact of sodium percarbonate stabilization processes is a critical consideration in the development and implementation of innovative techniques. These processes, while essential for maintaining the efficacy of sodium percarbonate as a bleaching and cleaning agent, can have significant implications for the environment.
One of the primary environmental concerns associated with stabilization processes is the use of chemical additives. Many traditional stabilization techniques rely on the incorporation of heavy metals, such as copper, manganese, or silver compounds. These metals, while effective in enhancing the stability of sodium percarbonate, can accumulate in soil and water systems, potentially leading to long-term ecological damage and bioaccumulation in various organisms.
Water consumption and wastewater generation are also notable environmental factors in stabilization processes. Some techniques require substantial amounts of water for coating or encapsulation procedures, contributing to water scarcity issues in regions where water resources are already strained. Additionally, the wastewater produced during these processes may contain residual chemicals and particulates that require proper treatment before discharge to prevent water pollution.
Energy usage is another significant environmental aspect of stabilization processes. Many techniques involve heating, drying, or other energy-intensive steps that contribute to greenhouse gas emissions when powered by non-renewable energy sources. The carbon footprint associated with these processes can be substantial, particularly in large-scale industrial applications.
Recent innovations in sodium percarbonate stabilization have begun to address these environmental concerns. For instance, the development of bio-based stabilizers derived from natural sources offers a more environmentally friendly alternative to traditional chemical additives. These organic stabilizers are often biodegradable and pose less risk of environmental accumulation.
Advancements in process efficiency have also led to reduced water and energy consumption. Techniques such as fluid bed coating and spray drying have been optimized to minimize resource use while maintaining or improving stabilization effectiveness. Furthermore, the integration of closed-loop systems and water recycling technologies has significantly reduced wastewater generation in some stabilization processes.
The shift towards greener stabilization methods has also seen the adoption of renewable energy sources in production facilities. Solar and wind power are increasingly being utilized to power stabilization processes, reducing the overall carbon footprint of sodium percarbonate production.
As environmental regulations become more stringent globally, there is a growing emphasis on life cycle assessments of stabilization processes. These comprehensive evaluations consider the environmental impact from raw material extraction through production, use, and disposal, driving further innovations in sustainable stabilization techniques.
One of the primary environmental concerns associated with stabilization processes is the use of chemical additives. Many traditional stabilization techniques rely on the incorporation of heavy metals, such as copper, manganese, or silver compounds. These metals, while effective in enhancing the stability of sodium percarbonate, can accumulate in soil and water systems, potentially leading to long-term ecological damage and bioaccumulation in various organisms.
Water consumption and wastewater generation are also notable environmental factors in stabilization processes. Some techniques require substantial amounts of water for coating or encapsulation procedures, contributing to water scarcity issues in regions where water resources are already strained. Additionally, the wastewater produced during these processes may contain residual chemicals and particulates that require proper treatment before discharge to prevent water pollution.
Energy usage is another significant environmental aspect of stabilization processes. Many techniques involve heating, drying, or other energy-intensive steps that contribute to greenhouse gas emissions when powered by non-renewable energy sources. The carbon footprint associated with these processes can be substantial, particularly in large-scale industrial applications.
Recent innovations in sodium percarbonate stabilization have begun to address these environmental concerns. For instance, the development of bio-based stabilizers derived from natural sources offers a more environmentally friendly alternative to traditional chemical additives. These organic stabilizers are often biodegradable and pose less risk of environmental accumulation.
Advancements in process efficiency have also led to reduced water and energy consumption. Techniques such as fluid bed coating and spray drying have been optimized to minimize resource use while maintaining or improving stabilization effectiveness. Furthermore, the integration of closed-loop systems and water recycling technologies has significantly reduced wastewater generation in some stabilization processes.
The shift towards greener stabilization methods has also seen the adoption of renewable energy sources in production facilities. Solar and wind power are increasingly being utilized to power stabilization processes, reducing the overall carbon footprint of sodium percarbonate production.
As environmental regulations become more stringent globally, there is a growing emphasis on life cycle assessments of stabilization processes. These comprehensive evaluations consider the environmental impact from raw material extraction through production, use, and disposal, driving further innovations in sustainable stabilization techniques.
Regulatory Framework for Percarbonate-based Products
The regulatory framework for percarbonate-based products is a critical aspect of the sodium percarbonate industry, encompassing various guidelines and standards that govern the production, distribution, and use of these products. In the United States, the Environmental Protection Agency (EPA) plays a pivotal role in regulating percarbonate-based products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This act requires manufacturers to register their products and provide detailed information on their composition, efficacy, and potential environmental impacts.
The European Union has established its own set of regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program. REACH mandates that companies producing or importing percarbonate-based products in quantities exceeding one ton per year must register these substances with the European Chemicals Agency (ECHA). This registration process involves submitting comprehensive data on the chemical properties, potential risks, and safe handling procedures of the products.
In addition to these overarching regulatory frameworks, individual countries often have their own specific requirements for percarbonate-based products. For instance, Japan's Ministry of Health, Labour and Welfare has established guidelines for the use of sodium percarbonate in household cleaning products, focusing on safety assessments and labeling requirements.
The regulatory landscape also extends to the transportation of sodium percarbonate. The International Maritime Dangerous Goods (IMDG) Code classifies sodium percarbonate as a Class 5.1 oxidizing substance, necessitating specific packaging, labeling, and handling procedures during shipping. Similarly, air transport is governed by the International Air Transport Association (IATA) Dangerous Goods Regulations, which outline strict guidelines for the carriage of percarbonate-based products.
Manufacturers must also adhere to workplace safety regulations when producing sodium percarbonate. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits and safety protocols for workers handling this substance. These regulations encompass aspects such as personal protective equipment, ventilation requirements, and emergency response procedures.
As environmental concerns continue to grow, regulatory bodies are increasingly focusing on the ecological impact of percarbonate-based products. This has led to the development of eco-labeling schemes and certification programs that assess the environmental performance of these products throughout their lifecycle. Manufacturers seeking to market their products as environmentally friendly must meet stringent criteria set by organizations such as the EU Ecolabel or the US EPA's Safer Choice program.
The European Union has established its own set of regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program. REACH mandates that companies producing or importing percarbonate-based products in quantities exceeding one ton per year must register these substances with the European Chemicals Agency (ECHA). This registration process involves submitting comprehensive data on the chemical properties, potential risks, and safe handling procedures of the products.
In addition to these overarching regulatory frameworks, individual countries often have their own specific requirements for percarbonate-based products. For instance, Japan's Ministry of Health, Labour and Welfare has established guidelines for the use of sodium percarbonate in household cleaning products, focusing on safety assessments and labeling requirements.
The regulatory landscape also extends to the transportation of sodium percarbonate. The International Maritime Dangerous Goods (IMDG) Code classifies sodium percarbonate as a Class 5.1 oxidizing substance, necessitating specific packaging, labeling, and handling procedures during shipping. Similarly, air transport is governed by the International Air Transport Association (IATA) Dangerous Goods Regulations, which outline strict guidelines for the carriage of percarbonate-based products.
Manufacturers must also adhere to workplace safety regulations when producing sodium percarbonate. In the United States, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits and safety protocols for workers handling this substance. These regulations encompass aspects such as personal protective equipment, ventilation requirements, and emergency response procedures.
As environmental concerns continue to grow, regulatory bodies are increasingly focusing on the ecological impact of percarbonate-based products. This has led to the development of eco-labeling schemes and certification programs that assess the environmental performance of these products throughout their lifecycle. Manufacturers seeking to market their products as environmentally friendly must meet stringent criteria set by organizations such as the EU Ecolabel or the US EPA's Safer Choice program.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!