Insights into Carbon Tetrachloride's Historical Industry Utilization
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Historical Use
Carbon tetrachloride (CCl4) has a rich history of industrial utilization dating back to the late 19th century. Initially discovered in 1839 by Henri Victor Regnault, it wasn't until the 1890s that CCl4 found widespread commercial applications. The compound's unique properties, including its non-flammability and excellent solvency, made it an attractive option for various industrial processes.
One of the earliest and most significant uses of carbon tetrachloride was as a dry-cleaning agent. Its ability to dissolve grease and oils without damaging fabrics made it a popular choice in the textile industry. This application continued well into the mid-20th century, with CCl4 being a staple in dry-cleaning establishments worldwide.
In the realm of fire safety, carbon tetrachloride played a crucial role. It was widely used in fire extinguishers, particularly in the early to mid-1900s. The compound's effectiveness in suppressing fires, especially those involving electrical equipment, made it a preferred choice for both industrial and domestic fire safety applications.
The chemical industry also found numerous uses for CCl4. It served as an important intermediate in the production of chlorofluorocarbons (CFCs), which were extensively used as refrigerants, propellants, and solvents. This application gained significant traction in the 1930s with the development of Freon and similar compounds.
In agriculture, carbon tetrachloride was employed as a fumigant for grain storage. Its ability to effectively eliminate pests made it a valuable tool in preserving harvested crops. This use was particularly prevalent in the mid-20th century before the environmental impacts of CCl4 were fully understood.
The manufacturing sector utilized carbon tetrachloride in various processes. It was used as a degreasing agent in metal cleaning operations, as a solvent in the production of semiconductors, and in the manufacture of certain plastics and synthetic fibers. Its low reactivity and excellent solvency properties made it ideal for these applications.
However, the widespread use of carbon tetrachloride began to decline in the latter half of the 20th century. Growing awareness of its toxicity and its role in ozone depletion led to increased regulation and eventual phase-out in many applications. The Montreal Protocol, signed in 1987, marked a turning point in the industrial use of CCl4, leading to its gradual elimination in most developed countries.
Despite its decline, the historical use of carbon tetrachloride has left a lasting impact on industrial practices and environmental policies. Its legacy serves as a reminder of the need for careful evaluation of chemical compounds in industrial applications and the importance of considering long-term environmental consequences.
One of the earliest and most significant uses of carbon tetrachloride was as a dry-cleaning agent. Its ability to dissolve grease and oils without damaging fabrics made it a popular choice in the textile industry. This application continued well into the mid-20th century, with CCl4 being a staple in dry-cleaning establishments worldwide.
In the realm of fire safety, carbon tetrachloride played a crucial role. It was widely used in fire extinguishers, particularly in the early to mid-1900s. The compound's effectiveness in suppressing fires, especially those involving electrical equipment, made it a preferred choice for both industrial and domestic fire safety applications.
The chemical industry also found numerous uses for CCl4. It served as an important intermediate in the production of chlorofluorocarbons (CFCs), which were extensively used as refrigerants, propellants, and solvents. This application gained significant traction in the 1930s with the development of Freon and similar compounds.
In agriculture, carbon tetrachloride was employed as a fumigant for grain storage. Its ability to effectively eliminate pests made it a valuable tool in preserving harvested crops. This use was particularly prevalent in the mid-20th century before the environmental impacts of CCl4 were fully understood.
The manufacturing sector utilized carbon tetrachloride in various processes. It was used as a degreasing agent in metal cleaning operations, as a solvent in the production of semiconductors, and in the manufacture of certain plastics and synthetic fibers. Its low reactivity and excellent solvency properties made it ideal for these applications.
However, the widespread use of carbon tetrachloride began to decline in the latter half of the 20th century. Growing awareness of its toxicity and its role in ozone depletion led to increased regulation and eventual phase-out in many applications. The Montreal Protocol, signed in 1987, marked a turning point in the industrial use of CCl4, leading to its gradual elimination in most developed countries.
Despite its decline, the historical use of carbon tetrachloride has left a lasting impact on industrial practices and environmental policies. Its legacy serves as a reminder of the need for careful evaluation of chemical compounds in industrial applications and the importance of considering long-term environmental consequences.
Market Demand Analysis
Carbon tetrachloride, once a widely used industrial chemical, has experienced significant shifts in market demand over the years. Initially, its versatile properties made it a popular choice across various industries. In the early to mid-20th century, the demand for carbon tetrachloride was primarily driven by its applications as a cleaning agent, solvent, and fire extinguishing agent.
The chemical industry heavily relied on carbon tetrachloride for the production of chlorofluorocarbons (CFCs), which were extensively used in refrigerants and aerosol propellants. This application created a substantial market for carbon tetrachloride, with production volumes reaching their peak in the 1970s. The automotive sector also contributed to the demand, utilizing carbon tetrachloride in the manufacturing of antiknock agents for gasoline.
However, the market landscape began to change dramatically in the late 1970s and early 1980s. Growing awareness of the environmental and health risks associated with carbon tetrachloride led to a sharp decline in demand. The Montreal Protocol, signed in 1987, aimed to phase out substances that deplete the ozone layer, including CFCs. This international agreement had a profound impact on the carbon tetrachloride market, as it effectively eliminated one of its primary uses.
The decline in demand was further accelerated by the development of safer alternatives for many of carbon tetrachloride's applications. Industries began to shift towards more environmentally friendly and less toxic substances for cleaning, fire suppression, and as solvents. This transition was driven by both regulatory pressures and growing consumer awareness of environmental issues.
Despite the overall decline, some niche markets for carbon tetrachloride persisted. The pharmaceutical industry continued to use small quantities of the chemical in the synthesis of certain drugs. Additionally, some specialized industrial processes still relied on carbon tetrachloride, albeit in much smaller volumes compared to its peak usage.
In recent years, the global market for carbon tetrachloride has stabilized at a much lower level than its historical highs. The remaining demand is primarily from countries with less stringent environmental regulations or those that have exemptions for specific critical uses. The future market trajectory for carbon tetrachloride is expected to continue its downward trend as more countries implement stricter environmental policies and as research into safer alternatives progresses.
The chemical industry heavily relied on carbon tetrachloride for the production of chlorofluorocarbons (CFCs), which were extensively used in refrigerants and aerosol propellants. This application created a substantial market for carbon tetrachloride, with production volumes reaching their peak in the 1970s. The automotive sector also contributed to the demand, utilizing carbon tetrachloride in the manufacturing of antiknock agents for gasoline.
However, the market landscape began to change dramatically in the late 1970s and early 1980s. Growing awareness of the environmental and health risks associated with carbon tetrachloride led to a sharp decline in demand. The Montreal Protocol, signed in 1987, aimed to phase out substances that deplete the ozone layer, including CFCs. This international agreement had a profound impact on the carbon tetrachloride market, as it effectively eliminated one of its primary uses.
The decline in demand was further accelerated by the development of safer alternatives for many of carbon tetrachloride's applications. Industries began to shift towards more environmentally friendly and less toxic substances for cleaning, fire suppression, and as solvents. This transition was driven by both regulatory pressures and growing consumer awareness of environmental issues.
Despite the overall decline, some niche markets for carbon tetrachloride persisted. The pharmaceutical industry continued to use small quantities of the chemical in the synthesis of certain drugs. Additionally, some specialized industrial processes still relied on carbon tetrachloride, albeit in much smaller volumes compared to its peak usage.
In recent years, the global market for carbon tetrachloride has stabilized at a much lower level than its historical highs. The remaining demand is primarily from countries with less stringent environmental regulations or those that have exemptions for specific critical uses. The future market trajectory for carbon tetrachloride is expected to continue its downward trend as more countries implement stricter environmental policies and as research into safer alternatives progresses.
Technical Challenges
Carbon tetrachloride's historical industry utilization faced several significant technical challenges that ultimately led to its decline and eventual phase-out in many applications. One of the primary obstacles was its high toxicity and potential carcinogenicity, which posed severe health risks to workers and consumers exposed to the substance. This necessitated the development of stringent safety protocols and protective equipment, increasing production costs and limiting its widespread use.
Another major challenge was carbon tetrachloride's ozone-depleting properties. As scientific understanding of atmospheric chemistry advanced, it became clear that the release of carbon tetrachloride into the atmosphere was contributing to the depletion of the ozone layer. This realization led to international regulations and agreements, such as the Montreal Protocol, which aimed to phase out the production and use of ozone-depleting substances.
The corrosive nature of carbon tetrachloride presented additional technical difficulties in its handling and storage. It required specialized equipment and materials that could withstand its corrosive effects, further increasing the costs associated with its use. This corrosivity also limited its application in certain industries where material compatibility was crucial.
Environmental persistence was another significant challenge. Carbon tetrachloride's stability in the environment meant that it could accumulate in soil and water systems, potentially causing long-term ecological damage. This persistence made remediation efforts complex and expensive, creating additional liabilities for industries that had historically used the substance.
The flammability of carbon tetrachloride vapors posed fire and explosion risks in industrial settings. While the liquid itself is not highly flammable, its vapors could form explosive mixtures with air under certain conditions. This required the implementation of specialized fire safety measures and ventilation systems, adding to the complexity of its industrial use.
As environmental and health regulations became more stringent, industries faced increasing pressure to find alternatives to carbon tetrachloride. This transition presented its own set of technical challenges, as replacement substances often had different properties and required modifications to existing processes and equipment. The search for suitable substitutes that could match carbon tetrachloride's performance in various applications, such as cleaning and degreasing, proved to be a significant technological hurdle.
Lastly, the disposal of carbon tetrachloride and contaminated materials presented ongoing challenges. Its resistance to degradation made conventional waste treatment methods ineffective, necessitating the development of specialized disposal techniques. This added to the overall cost and complexity of using carbon tetrachloride in industrial processes, further driving the need for alternative solutions.
Another major challenge was carbon tetrachloride's ozone-depleting properties. As scientific understanding of atmospheric chemistry advanced, it became clear that the release of carbon tetrachloride into the atmosphere was contributing to the depletion of the ozone layer. This realization led to international regulations and agreements, such as the Montreal Protocol, which aimed to phase out the production and use of ozone-depleting substances.
The corrosive nature of carbon tetrachloride presented additional technical difficulties in its handling and storage. It required specialized equipment and materials that could withstand its corrosive effects, further increasing the costs associated with its use. This corrosivity also limited its application in certain industries where material compatibility was crucial.
Environmental persistence was another significant challenge. Carbon tetrachloride's stability in the environment meant that it could accumulate in soil and water systems, potentially causing long-term ecological damage. This persistence made remediation efforts complex and expensive, creating additional liabilities for industries that had historically used the substance.
The flammability of carbon tetrachloride vapors posed fire and explosion risks in industrial settings. While the liquid itself is not highly flammable, its vapors could form explosive mixtures with air under certain conditions. This required the implementation of specialized fire safety measures and ventilation systems, adding to the complexity of its industrial use.
As environmental and health regulations became more stringent, industries faced increasing pressure to find alternatives to carbon tetrachloride. This transition presented its own set of technical challenges, as replacement substances often had different properties and required modifications to existing processes and equipment. The search for suitable substitutes that could match carbon tetrachloride's performance in various applications, such as cleaning and degreasing, proved to be a significant technological hurdle.
Lastly, the disposal of carbon tetrachloride and contaminated materials presented ongoing challenges. Its resistance to degradation made conventional waste treatment methods ineffective, necessitating the development of specialized disposal techniques. This added to the overall cost and complexity of using carbon tetrachloride in industrial processes, further driving the need for alternative solutions.
Current Utilization
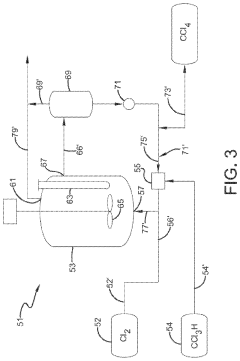
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical reactions, distillation processes, and other purification techniques to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes studies on its effects on the ozone layer and strategies for reducing its use in industrial processes.
- Detection and analysis methods: Various techniques and apparatus have been developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors designed to measure carbon tetrachloride concentrations in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past, including its use in fire extinguishers, dry cleaning, and as a refrigerant. These historical patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and synthetic reactions.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and potential health hazards, there are methods and systems developed for the safe handling, storage, and disposal of carbon tetrachloride. This includes techniques for detecting leaks, minimizing exposure, and treating contaminated areas.Expand Specific Solutions04 Alternatives and replacements for carbon tetrachloride
Research into alternatives and replacements for carbon tetrachloride in various applications, aiming to reduce its use due to environmental and health concerns. This includes developing new compounds or processes that can perform similar functions with reduced risks.Expand Specific Solutions05 Historical uses and early patents related to carbon tetrachloride
Early patents and historical uses of carbon tetrachloride, including its applications in fire extinguishers, dry cleaning, and as a refrigerant. These documents provide insight into the compound's discovery and initial industrial applications.Expand Specific Solutions
Key Industry Players
The historical utilization of carbon tetrachloride in industry has evolved through various stages, reflecting changes in market demands and regulatory environments. Initially widely used in manufacturing and as a solvent, its market size has significantly decreased due to environmental and health concerns. The technology's maturity has shifted from widespread industrial application to more specialized and controlled uses. Companies like DuPont de Nemours, Inc. and Occidental Chemical Corp. have played key roles in its production and application, while others such as Tronox LLC and The Chemours Co. have been involved in related chemical industries. Research institutions like Central South University and the University of Florida have contributed to understanding its properties and environmental impact, influencing its current limited use.
DuPont de Nemours, Inc.
Technical Solution: DuPont has a long history of involvement with carbon tetrachloride (CCl4) in industrial applications. The company developed and utilized CCl4 as a key ingredient in the production of chlorofluorocarbons (CFCs), particularly in the manufacture of refrigerants and aerosol propellants [1]. DuPont's technical approach involved using CCl4 as a feedstock in the synthesis of CFCs, where it reacted with hydrogen fluoride to produce various CFC compounds [2]. The company also employed CCl4 as a solvent in various industrial processes, including dry cleaning and metal degreasing [3]. DuPont's expertise in handling and processing CCl4 led to the development of safety protocols and specialized equipment for its use in industrial settings.
Strengths: Extensive experience in CCl4 handling and processing; developed efficient CFC production methods. Weaknesses: Phaseout of CFCs due to environmental concerns has significantly reduced CCl4 usage; potential environmental liabilities from historical use.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. (OxyChem) has been a significant player in the chlor-alkali industry, which historically involved the production and use of carbon tetrachloride. OxyChem's technical approach to CCl4 utilization focused on its role as an intermediate in the production of perchloroethylene and other chlorinated solvents [4]. The company developed processes that used CCl4 in the manufacture of these products, employing high-temperature chlorination reactions [5]. OxyChem also explored the use of CCl4 in the production of chlorofluorocarbons before their phaseout. The company's expertise extended to the development of containment and handling systems for CCl4, given its toxicity and environmental concerns [6].
Strengths: Established infrastructure for chlorinated solvent production; expertise in handling hazardous chlorinated compounds. Weaknesses: Declining market for CCl4-derived products; need for significant investment in alternative technologies.
Innovative Applications
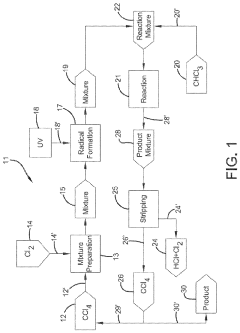
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
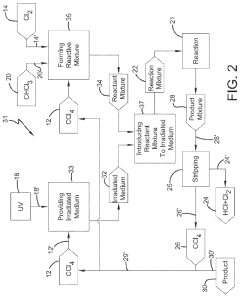
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact
The historical utilization of carbon tetrachloride in various industries has left a significant environmental footprint, with far-reaching consequences for ecosystems and human health. As a potent ozone-depleting substance, carbon tetrachloride has contributed to the depletion of the Earth's protective ozone layer, leading to increased ultraviolet radiation reaching the planet's surface. This has resulted in adverse effects on terrestrial and aquatic ecosystems, including reduced plant growth, altered marine food chains, and increased susceptibility of organisms to UV-related damage.
In aquatic environments, carbon tetrachloride contamination has been particularly problematic. Its high density and low water solubility have caused it to sink and accumulate in sediments, creating long-lasting pollution sources in rivers, lakes, and coastal areas. This persistence has led to bioaccumulation in aquatic organisms, with potential impacts on entire food webs and human consumers of seafood.
Soil contamination from industrial spills and improper disposal practices has been another major environmental concern. Carbon tetrachloride's mobility in soil has resulted in widespread groundwater contamination, affecting drinking water sources in many areas. The compound's resistance to natural degradation processes has made remediation efforts challenging and costly, often requiring long-term monitoring and treatment strategies.
Air pollution from carbon tetrachloride emissions has not only contributed to ozone depletion but also posed direct health risks to exposed populations. Indoor air quality has been particularly affected in areas where the compound was used in dry cleaning, fire extinguishers, or as a solvent in various industrial processes. The volatility of carbon tetrachloride has led to its widespread distribution in the atmosphere, making it a global environmental pollutant.
The environmental impact of carbon tetrachloride has also extended to climate change. As a greenhouse gas, it has contributed to global warming, albeit to a lesser extent than carbon dioxide or methane. However, its long atmospheric lifetime means that its climate effects persist for many decades after emission.
Efforts to mitigate the environmental impact of carbon tetrachloride have included international regulations such as the Montreal Protocol, which phased out its production and use in many applications. However, the legacy of past use continues to pose environmental challenges, necessitating ongoing remediation efforts and environmental monitoring. The experience with carbon tetrachloride serves as a cautionary tale, highlighting the importance of thorough environmental impact assessments before widespread industrial adoption of chemical compounds.
In aquatic environments, carbon tetrachloride contamination has been particularly problematic. Its high density and low water solubility have caused it to sink and accumulate in sediments, creating long-lasting pollution sources in rivers, lakes, and coastal areas. This persistence has led to bioaccumulation in aquatic organisms, with potential impacts on entire food webs and human consumers of seafood.
Soil contamination from industrial spills and improper disposal practices has been another major environmental concern. Carbon tetrachloride's mobility in soil has resulted in widespread groundwater contamination, affecting drinking water sources in many areas. The compound's resistance to natural degradation processes has made remediation efforts challenging and costly, often requiring long-term monitoring and treatment strategies.
Air pollution from carbon tetrachloride emissions has not only contributed to ozone depletion but also posed direct health risks to exposed populations. Indoor air quality has been particularly affected in areas where the compound was used in dry cleaning, fire extinguishers, or as a solvent in various industrial processes. The volatility of carbon tetrachloride has led to its widespread distribution in the atmosphere, making it a global environmental pollutant.
The environmental impact of carbon tetrachloride has also extended to climate change. As a greenhouse gas, it has contributed to global warming, albeit to a lesser extent than carbon dioxide or methane. However, its long atmospheric lifetime means that its climate effects persist for many decades after emission.
Efforts to mitigate the environmental impact of carbon tetrachloride have included international regulations such as the Montreal Protocol, which phased out its production and use in many applications. However, the legacy of past use continues to pose environmental challenges, necessitating ongoing remediation efforts and environmental monitoring. The experience with carbon tetrachloride serves as a cautionary tale, highlighting the importance of thorough environmental impact assessments before widespread industrial adoption of chemical compounds.
Regulatory Framework
The regulatory framework surrounding carbon tetrachloride has evolved significantly over the years, reflecting growing awareness of its environmental and health impacts. In the early 20th century, when carbon tetrachloride was widely used in various industries, there were minimal regulations governing its production, use, and disposal. However, as scientific evidence emerged about its harmful effects, governments and international organizations began implementing stricter controls.
The Montreal Protocol, signed in 1987, marked a turning point in the regulation of carbon tetrachloride. This international treaty aimed to phase out substances that deplete the ozone layer, including carbon tetrachloride. Signatories agreed to gradually reduce and eventually eliminate the production and consumption of ozone-depleting substances. As a result, many countries implemented national laws and regulations to comply with the protocol's requirements.
In the United States, the Environmental Protection Agency (EPA) has played a crucial role in regulating carbon tetrachloride. The Toxic Substances Control Act (TSCA) of 1976 gave the EPA authority to restrict the use of chemicals that pose unreasonable risks to human health or the environment. Under this act, the EPA has imposed strict limitations on the production and use of carbon tetrachloride.
The Clean Air Act Amendments of 1990 further strengthened regulations by classifying carbon tetrachloride as a hazardous air pollutant. This classification led to more stringent emission controls and monitoring requirements for industries that still used or produced the chemical. Additionally, the Resource Conservation and Recovery Act (RCRA) established guidelines for the proper handling and disposal of hazardous waste, including carbon tetrachloride-containing materials.
Internationally, the European Union has implemented its own set of regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program. REACH requires companies to register chemical substances and provide safety information, with carbon tetrachloride subject to strict authorization requirements due to its high concern status.
Despite these regulations, carbon tetrachloride continues to be produced in small quantities for specific exempted uses, such as feedstock for the production of other chemicals. However, these exemptions are closely monitored and subject to periodic review. The regulatory framework also includes reporting requirements, with many countries mandating regular assessments of carbon tetrachloride emissions and stockpiles.
As scientific understanding of the long-term environmental impacts of carbon tetrachloride continues to grow, regulatory bodies remain vigilant, periodically updating their policies to address emerging concerns and ensure the protection of human health and the environment.
The Montreal Protocol, signed in 1987, marked a turning point in the regulation of carbon tetrachloride. This international treaty aimed to phase out substances that deplete the ozone layer, including carbon tetrachloride. Signatories agreed to gradually reduce and eventually eliminate the production and consumption of ozone-depleting substances. As a result, many countries implemented national laws and regulations to comply with the protocol's requirements.
In the United States, the Environmental Protection Agency (EPA) has played a crucial role in regulating carbon tetrachloride. The Toxic Substances Control Act (TSCA) of 1976 gave the EPA authority to restrict the use of chemicals that pose unreasonable risks to human health or the environment. Under this act, the EPA has imposed strict limitations on the production and use of carbon tetrachloride.
The Clean Air Act Amendments of 1990 further strengthened regulations by classifying carbon tetrachloride as a hazardous air pollutant. This classification led to more stringent emission controls and monitoring requirements for industries that still used or produced the chemical. Additionally, the Resource Conservation and Recovery Act (RCRA) established guidelines for the proper handling and disposal of hazardous waste, including carbon tetrachloride-containing materials.
Internationally, the European Union has implemented its own set of regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program. REACH requires companies to register chemical substances and provide safety information, with carbon tetrachloride subject to strict authorization requirements due to its high concern status.
Despite these regulations, carbon tetrachloride continues to be produced in small quantities for specific exempted uses, such as feedstock for the production of other chemicals. However, these exemptions are closely monitored and subject to periodic review. The regulatory framework also includes reporting requirements, with many countries mandating regular assessments of carbon tetrachloride emissions and stockpiles.
As scientific understanding of the long-term environmental impacts of carbon tetrachloride continues to grow, regulatory bodies remain vigilant, periodically updating their policies to address emerging concerns and ensure the protection of human health and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!