Potential of Fluoroantimonic Acid in Battery Technologies
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid in Batteries: Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride and antimony pentafluoride, has recently garnered attention in the field of battery technologies. This powerful acid, known for its extreme corrosiveness and ability to protonate even weak bases, presents intriguing possibilities for enhancing battery performance and efficiency.
The development of advanced battery technologies has been a critical focus in the energy sector for decades. As the world transitions towards renewable energy sources and electric vehicles, the demand for high-performance, long-lasting, and fast-charging batteries continues to grow. In this context, researchers and industry experts are constantly exploring novel materials and chemical compounds that could potentially revolutionize battery design and functionality.
Fluoroantimonic acid's unique properties make it an interesting candidate for battery applications. Its exceptionally high acidity, with a Hammett acidity function estimated at -28, surpasses that of conventional battery electrolytes. This extreme acidity could potentially lead to enhanced ion conductivity and improved electrode-electrolyte interactions, addressing some of the key challenges in current battery technologies.
The exploration of fluoroantimonic acid in battery systems aligns with the broader trend of investigating super- and hyperacid electrolytes. These advanced electrolytes have shown promise in improving various aspects of battery performance, including energy density, power output, and cycling stability. By leveraging the unique chemical properties of fluoroantimonic acid, researchers aim to push the boundaries of what is possible in energy storage devices.
However, the integration of fluoroantimonic acid into battery technologies is not without significant challenges. Its highly corrosive nature poses substantial safety and material compatibility issues that must be carefully addressed. The development of suitable containment materials and safety protocols is crucial for any practical application of this superacid in battery systems.
The primary objectives of investigating fluoroantimonic acid in battery technologies are multifaceted. Researchers aim to explore its potential for enhancing electrolyte conductivity, improving electrode-electrolyte interfaces, and possibly enabling new electrode materials or battery chemistries. Additionally, there is interest in understanding how the extreme acidity of fluoroantimonic acid could be harnessed to overcome limitations in current battery designs, such as dendrite formation in lithium-metal batteries or capacity fading in other advanced battery systems.
As research in this area progresses, it is essential to consider the broader implications of incorporating such a powerful acid into energy storage devices. This includes evaluating the environmental impact, assessing the scalability of production, and ensuring that any benefits in performance outweigh the potential risks and challenges associated with its use.
The development of advanced battery technologies has been a critical focus in the energy sector for decades. As the world transitions towards renewable energy sources and electric vehicles, the demand for high-performance, long-lasting, and fast-charging batteries continues to grow. In this context, researchers and industry experts are constantly exploring novel materials and chemical compounds that could potentially revolutionize battery design and functionality.
Fluoroantimonic acid's unique properties make it an interesting candidate for battery applications. Its exceptionally high acidity, with a Hammett acidity function estimated at -28, surpasses that of conventional battery electrolytes. This extreme acidity could potentially lead to enhanced ion conductivity and improved electrode-electrolyte interactions, addressing some of the key challenges in current battery technologies.
The exploration of fluoroantimonic acid in battery systems aligns with the broader trend of investigating super- and hyperacid electrolytes. These advanced electrolytes have shown promise in improving various aspects of battery performance, including energy density, power output, and cycling stability. By leveraging the unique chemical properties of fluoroantimonic acid, researchers aim to push the boundaries of what is possible in energy storage devices.
However, the integration of fluoroantimonic acid into battery technologies is not without significant challenges. Its highly corrosive nature poses substantial safety and material compatibility issues that must be carefully addressed. The development of suitable containment materials and safety protocols is crucial for any practical application of this superacid in battery systems.
The primary objectives of investigating fluoroantimonic acid in battery technologies are multifaceted. Researchers aim to explore its potential for enhancing electrolyte conductivity, improving electrode-electrolyte interfaces, and possibly enabling new electrode materials or battery chemistries. Additionally, there is interest in understanding how the extreme acidity of fluoroantimonic acid could be harnessed to overcome limitations in current battery designs, such as dendrite formation in lithium-metal batteries or capacity fading in other advanced battery systems.
As research in this area progresses, it is essential to consider the broader implications of incorporating such a powerful acid into energy storage devices. This includes evaluating the environmental impact, assessing the scalability of production, and ensuring that any benefits in performance outweigh the potential risks and challenges associated with its use.
Market Analysis for Advanced Battery Technologies
The advanced battery technologies market is experiencing rapid growth and transformation, driven by increasing demand for electric vehicles, renewable energy storage, and portable electronic devices. This market segment is expected to continue its upward trajectory, with projections indicating substantial expansion over the next decade.
The global advanced battery market is primarily segmented into lithium-ion, lead-acid, nickel-metal hydride, and emerging technologies such as solid-state batteries. Among these, lithium-ion batteries currently dominate the market due to their high energy density, long cycle life, and decreasing costs. However, the potential introduction of fluoroantimonic acid in battery technologies could disrupt this landscape.
Electric vehicles represent a significant driver for advanced battery technologies. As governments worldwide implement stricter emissions regulations and offer incentives for EV adoption, the demand for high-performance, long-range batteries continues to surge. This trend is expected to accelerate, with major automakers committing to electrify their vehicle lineups in the coming years.
The renewable energy sector is another key market for advanced batteries. As wind and solar power generation increases, the need for efficient and large-scale energy storage solutions grows proportionally. Grid-scale battery storage systems are becoming increasingly crucial for balancing supply and demand, ensuring grid stability, and enabling the wider adoption of renewable energy sources.
Consumer electronics, including smartphones, laptops, and wearable devices, constitute another significant market segment for advanced batteries. The continuous evolution of these devices, with increasing power requirements and decreasing form factors, drives the demand for batteries with higher energy density and faster charging capabilities.
The potential introduction of fluoroantimonic acid in battery technologies could address several key challenges in the current market. Its super-acidic properties might enable the development of batteries with significantly higher energy density, potentially revolutionizing electric vehicle range and portable device battery life. Additionally, it could lead to faster charging times and improved safety features, addressing critical consumer concerns.
However, the market adoption of fluoroantimonic acid-based batteries would face several hurdles. These include the need for extensive research and development, safety considerations due to the highly corrosive nature of the acid, and the establishment of new manufacturing processes and supply chains. The success of this technology would also depend on its cost-effectiveness compared to existing and emerging battery technologies.
The global advanced battery market is primarily segmented into lithium-ion, lead-acid, nickel-metal hydride, and emerging technologies such as solid-state batteries. Among these, lithium-ion batteries currently dominate the market due to their high energy density, long cycle life, and decreasing costs. However, the potential introduction of fluoroantimonic acid in battery technologies could disrupt this landscape.
Electric vehicles represent a significant driver for advanced battery technologies. As governments worldwide implement stricter emissions regulations and offer incentives for EV adoption, the demand for high-performance, long-range batteries continues to surge. This trend is expected to accelerate, with major automakers committing to electrify their vehicle lineups in the coming years.
The renewable energy sector is another key market for advanced batteries. As wind and solar power generation increases, the need for efficient and large-scale energy storage solutions grows proportionally. Grid-scale battery storage systems are becoming increasingly crucial for balancing supply and demand, ensuring grid stability, and enabling the wider adoption of renewable energy sources.
Consumer electronics, including smartphones, laptops, and wearable devices, constitute another significant market segment for advanced batteries. The continuous evolution of these devices, with increasing power requirements and decreasing form factors, drives the demand for batteries with higher energy density and faster charging capabilities.
The potential introduction of fluoroantimonic acid in battery technologies could address several key challenges in the current market. Its super-acidic properties might enable the development of batteries with significantly higher energy density, potentially revolutionizing electric vehicle range and portable device battery life. Additionally, it could lead to faster charging times and improved safety features, addressing critical consumer concerns.
However, the market adoption of fluoroantimonic acid-based batteries would face several hurdles. These include the need for extensive research and development, safety considerations due to the highly corrosive nature of the acid, and the establishment of new manufacturing processes and supply chains. The success of this technology would also depend on its cost-effectiveness compared to existing and emerging battery technologies.
Current State and Challenges in Fluoroantimonic Acid Application
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various scientific fields, including battery technologies. However, its application in this domain faces several challenges and limitations that need to be addressed.
The current state of fluoroantimonic acid in battery technologies is primarily experimental, with limited practical applications due to its extreme reactivity and corrosive nature. Research has shown potential for its use as an electrolyte component in certain types of batteries, particularly those requiring high-energy density and rapid charge-discharge cycles.
One of the main challenges in utilizing fluoroantimonic acid is its extreme reactivity with most materials, including those commonly used in battery construction. This reactivity limits the choice of electrode materials and container materials, significantly constraining design options and increasing production costs.
Safety concerns pose another significant challenge. The highly corrosive nature of fluoroantimonic acid necessitates stringent safety protocols and specialized handling equipment, which can be prohibitively expensive for large-scale production. Additionally, the potential environmental impact of accidental releases is a major concern, requiring robust containment and disposal systems.
Stability issues also present a considerable obstacle. Fluoroantimonic acid is highly sensitive to moisture and can decompose rapidly when exposed to air, making long-term storage and use in batteries problematic. This instability affects the overall lifespan and reliability of battery systems incorporating this superacid.
From a manufacturing perspective, the production of high-purity fluoroantimonic acid at scale remains a challenge. Current synthesis methods are complex and expensive, limiting its availability for widespread use in battery technologies. This scarcity also contributes to the high cost of research and development in this area.
Despite these challenges, ongoing research continues to explore potential solutions. Some studies are focusing on developing novel containment materials that can withstand the acid's corrosive properties. Others are investigating ways to stabilize the acid or create less reactive derivatives that retain its beneficial properties for battery applications.
The regulatory landscape surrounding the use of fluoroantimonic acid in consumer products is another area of concern. Stringent safety regulations and potential environmental restrictions may limit its adoption in commercial battery technologies, necessitating extensive testing and approval processes.
In conclusion, while fluoroantimonic acid shows promise in enhancing battery performance, significant technological, safety, and regulatory hurdles must be overcome before it can be widely adopted in battery technologies. The current state of research suggests that practical applications may still be several years away, requiring continued innovation in materials science and chemical engineering.
The current state of fluoroantimonic acid in battery technologies is primarily experimental, with limited practical applications due to its extreme reactivity and corrosive nature. Research has shown potential for its use as an electrolyte component in certain types of batteries, particularly those requiring high-energy density and rapid charge-discharge cycles.
One of the main challenges in utilizing fluoroantimonic acid is its extreme reactivity with most materials, including those commonly used in battery construction. This reactivity limits the choice of electrode materials and container materials, significantly constraining design options and increasing production costs.
Safety concerns pose another significant challenge. The highly corrosive nature of fluoroantimonic acid necessitates stringent safety protocols and specialized handling equipment, which can be prohibitively expensive for large-scale production. Additionally, the potential environmental impact of accidental releases is a major concern, requiring robust containment and disposal systems.
Stability issues also present a considerable obstacle. Fluoroantimonic acid is highly sensitive to moisture and can decompose rapidly when exposed to air, making long-term storage and use in batteries problematic. This instability affects the overall lifespan and reliability of battery systems incorporating this superacid.
From a manufacturing perspective, the production of high-purity fluoroantimonic acid at scale remains a challenge. Current synthesis methods are complex and expensive, limiting its availability for widespread use in battery technologies. This scarcity also contributes to the high cost of research and development in this area.
Despite these challenges, ongoing research continues to explore potential solutions. Some studies are focusing on developing novel containment materials that can withstand the acid's corrosive properties. Others are investigating ways to stabilize the acid or create less reactive derivatives that retain its beneficial properties for battery applications.
The regulatory landscape surrounding the use of fluoroantimonic acid in consumer products is another area of concern. Stringent safety regulations and potential environmental restrictions may limit its adoption in commercial battery technologies, necessitating extensive testing and approval processes.
In conclusion, while fluoroantimonic acid shows promise in enhancing battery performance, significant technological, safety, and regulatory hurdles must be overcome before it can be widely adopted in battery technologies. The current state of research suggests that practical applications may still be several years away, requiring continued innovation in materials science and chemical engineering.
Existing Solutions Utilizing Fluoroantimonic Acid in Batteries
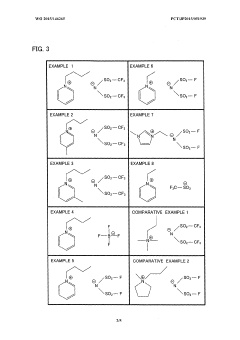
01 Synthesis and preparation of fluoroantimonic acid
Fluoroantimonic acid is synthesized by combining hydrogen fluoride and antimony pentafluoride. The process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the resulting superacid.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in catalysis and chemical reactions: Fluoroantimonic acid is a powerful superacid catalyst used in various chemical reactions. It is particularly effective in promoting alkylation, isomerization, and polymerization processes. The acid's extreme acidity allows it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in organic synthesis and petrochemical industries.
- Use in materials science and surface treatments: Fluoroantimonic acid finds applications in materials science, particularly in surface treatments and modifications. It can be used to etch or modify surfaces of various materials, including metals, semiconductors, and ceramics. The acid's unique properties allow for the creation of specialized surface structures and coatings with enhanced properties.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, fluoroantimonic acid requires specialized handling and storage procedures. Safety measures include the use of specialized containment materials, personal protective equipment, and strict protocols for handling and disposal. Research has been conducted to develop safer handling methods and to mitigate the risks associated with its use in industrial and laboratory settings.
- Analytical and characterization techniques: Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analyses, and computational modeling. Such techniques are crucial for understanding the acid's behavior, optimizing its use in different applications, and developing new methodologies for its production and utilization.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful catalyst in various organic synthesis reactions due to its extremely high acidity. It can catalyze alkylation, isomerization, and polymerization reactions. The superacid is particularly effective in promoting reactions that are difficult to achieve with conventional acid catalysts.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, and modify the properties of materials such as polymers and metals.Expand Specific Solutions04 Safety considerations and handling procedures
Due to its extreme corrosiveness and reactivity, special safety measures and handling procedures are required when working with fluoroantimonic acid. This includes the use of specialized containment materials, personal protective equipment, and strict protocols for storage, transport, and disposal.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analysis, and specialized apparatus for measuring superacidity. Such techniques are crucial for understanding the properties and behavior of this powerful superacid.Expand Specific Solutions
Key Players in Fluoroantimonic Acid and Battery Industries
The potential of fluoroantimonic acid in battery technologies is an emerging field in the early stages of development. The market size is currently limited, but growing interest from major automotive companies like Toyota and Honda suggests significant future potential. The technology's maturity is still low, with research primarily conducted by academic institutions such as California Institute of Technology and Huazhong University of Science & Technology. Industrial players like Panasonic Energy and Sanyo Chemical Industries are also exploring applications, indicating a gradual shift towards commercialization. However, the highly corrosive nature of fluoroantimonic acid presents significant challenges for practical implementation, necessitating further research and development to overcome safety and material compatibility issues.
Kanto Denka Kogyo Co., Ltd.
Technical Solution: Kanto Denka Kogyo has developed a novel approach to utilizing fluoroantimonic acid in battery technologies. Their method involves incorporating the superacid into a specialized polymer matrix, creating a highly conductive and stable electrolyte. This composite electrolyte allows for enhanced ion transport while mitigating the corrosive effects of the acid. The company has also developed a proprietary coating technique for electrode materials to protect them from degradation, enabling the use of high-energy density materials previously incompatible with such strong acids.
Strengths: Exceptional ionic conductivity, potential for higher energy density batteries. Weaknesses: Complexity in manufacturing, potential safety concerns due to the highly reactive nature of fluoroantimonic acid.
Panasonic Energy Co. Ltd.
Technical Solution: Panasonic Energy has been exploring the use of fluoroantimonic acid as a catalyst in the production of advanced electrode materials. Their approach involves using controlled amounts of the superacid to modify the surface structure of cathode materials, particularly for lithium-ion batteries. This process creates nanoscale defects and channels in the material, significantly improving lithium-ion diffusion and overall battery performance. Additionally, they are investigating the potential of fluoroantimonic acid in the recycling of spent batteries, using its strong acidic properties to efficiently separate and recover valuable materials.
Strengths: Improved electrode performance, potential for more efficient battery recycling. Weaknesses: High cost of implementation, stringent safety measures required in production.
Core Innovations in Fluoroantimonic Acid-Based Energy Storage
Liquid electrolyte for fluoride ion battery and fluoride ion battery
PatentWO2015146265A1
Innovation
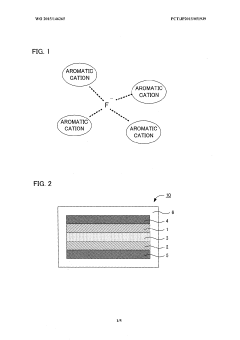
- A liquid electrolyte for fluoride ion batteries is developed using an aromatic solvent with an aromatic cation and anion, where the molar ratio of the aromatic cation to fluoride ions is greater than 1, enhancing the stability and reactivity of fluoride ions with active materials.
Fluoride ion battery compositions
PatentActiveUS20120164541A1
Innovation
- Development of a lithium-free, anion-based charge transport electrochemical system using fluoride ion transporting liquid electrolytes, specifically ionic liquids with organic-soluble fluoride salts and additives, which maintain conductivity at room and low temperatures, enabling efficient fluoride ion transfer between electrodes.
Safety and Handling Protocols for Fluoroantimonic Acid
Fluoroantimonic acid, known as the strongest superacid, requires stringent safety measures and handling protocols due to its extreme corrosiveness and reactivity. Proper personal protective equipment (PPE) is essential when working with this substance. This includes chemical-resistant suits, gloves, and boots made from materials such as fluorinated ethylene propylene (FEP) or polytetrafluoroethylene (PTFE). Full-face respirators with appropriate acid gas cartridges are necessary to protect against harmful vapors.
Storage of fluoroantimonic acid demands specialized containers made from materials resistant to its corrosive nature, such as PTFE or perfluoroalkoxy alkanes (PFA). These containers must be kept in a cool, dry, and well-ventilated area, away from incompatible materials like water, metals, and organic compounds. Regular inspections of storage facilities are crucial to detect any signs of container degradation or leaks.
Handling procedures for fluoroantimonic acid require a dedicated fume hood or glove box to contain and control any potential releases. All equipment used in handling this superacid must be thoroughly dried and free from moisture. Transfer operations should be conducted using specialized pumps and piping systems designed to withstand the acid's corrosive properties.
Emergency response protocols are critical when working with fluoroantimonic acid. Spill kits containing neutralizing agents such as sodium bicarbonate or calcium carbonate should be readily available. Personnel must be trained in proper spill containment and neutralization techniques. Eye wash stations and safety showers should be installed in close proximity to work areas.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization followed by proper disposal through licensed chemical waste handlers is essential. Any contaminated materials, including PPE, must be treated as hazardous waste and disposed of accordingly.
Training and education form a crucial component of safety protocols. All personnel working with or around fluoroantimonic acid must receive comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain a high level of awareness and preparedness.
Documentation and record-keeping are vital aspects of safety management. Detailed standard operating procedures (SOPs), safety data sheets (SDS), and risk assessments must be developed and regularly updated. Incident reporting systems should be in place to track and analyze any safety-related events, enabling continuous improvement of handling protocols.
Storage of fluoroantimonic acid demands specialized containers made from materials resistant to its corrosive nature, such as PTFE or perfluoroalkoxy alkanes (PFA). These containers must be kept in a cool, dry, and well-ventilated area, away from incompatible materials like water, metals, and organic compounds. Regular inspections of storage facilities are crucial to detect any signs of container degradation or leaks.
Handling procedures for fluoroantimonic acid require a dedicated fume hood or glove box to contain and control any potential releases. All equipment used in handling this superacid must be thoroughly dried and free from moisture. Transfer operations should be conducted using specialized pumps and piping systems designed to withstand the acid's corrosive properties.
Emergency response protocols are critical when working with fluoroantimonic acid. Spill kits containing neutralizing agents such as sodium bicarbonate or calcium carbonate should be readily available. Personnel must be trained in proper spill containment and neutralization techniques. Eye wash stations and safety showers should be installed in close proximity to work areas.
Waste disposal of fluoroantimonic acid and related materials requires careful consideration. Neutralization followed by proper disposal through licensed chemical waste handlers is essential. Any contaminated materials, including PPE, must be treated as hazardous waste and disposed of accordingly.
Training and education form a crucial component of safety protocols. All personnel working with or around fluoroantimonic acid must receive comprehensive training on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain a high level of awareness and preparedness.
Documentation and record-keeping are vital aspects of safety management. Detailed standard operating procedures (SOPs), safety data sheets (SDS), and risk assessments must be developed and regularly updated. Incident reporting systems should be in place to track and analyze any safety-related events, enabling continuous improvement of handling protocols.
Environmental Impact of Fluoroantimonic Acid in Batteries
The environmental impact of fluoroantimonic acid in batteries is a critical consideration for the development and implementation of this technology. Fluoroantimonic acid, known as one of the strongest superacids, poses significant environmental risks due to its highly corrosive and reactive nature.
When used in battery technologies, the primary environmental concern is the potential for acid leakage or spills during manufacturing, transportation, or disposal processes. Such incidents could lead to severe soil and water contamination, causing long-lasting damage to ecosystems and potentially harming human health. The acid's extreme reactivity with water makes it particularly dangerous if released into aquatic environments.
The production of fluoroantimonic acid involves the use of hydrofluoric acid and antimony pentafluoride, both of which have their own environmental implications. The manufacturing process may contribute to air pollution through the release of fluorine-containing compounds, which are known to have negative impacts on air quality and can contribute to the depletion of the ozone layer.
In terms of waste management, the disposal of batteries containing fluoroantimonic acid presents significant challenges. Conventional recycling methods may not be suitable due to the acid's extreme corrosiveness, potentially leading to increased hazardous waste generation. This could strain existing waste management infrastructure and increase the risk of environmental contamination.
The long-term environmental effects of fluoroantimonic acid exposure are not yet fully understood, but studies suggest potential bioaccumulation in food chains and persistent environmental contamination. This raises concerns about its impact on biodiversity and ecosystem stability over extended periods.
From a lifecycle perspective, the environmental footprint of fluoroantimonic acid-based batteries extends beyond their use phase. The extraction and processing of raw materials required for acid production, such as antimony and fluorine, can have substantial environmental impacts, including habitat destruction and resource depletion.
To mitigate these environmental risks, stringent safety protocols and containment measures would be necessary throughout the battery lifecycle. This includes robust packaging for transportation, secure storage facilities, and specialized handling procedures during manufacturing and recycling processes. Additionally, the development of effective neutralization and remediation techniques would be crucial for addressing potential spills or contamination incidents.
As the battery industry continues to explore innovative technologies, the environmental implications of fluoroantimonic acid must be carefully weighed against its potential benefits. Sustainable alternatives and improved safety measures should be actively researched to minimize the ecological footprint of this powerful but potentially hazardous substance in battery applications.
When used in battery technologies, the primary environmental concern is the potential for acid leakage or spills during manufacturing, transportation, or disposal processes. Such incidents could lead to severe soil and water contamination, causing long-lasting damage to ecosystems and potentially harming human health. The acid's extreme reactivity with water makes it particularly dangerous if released into aquatic environments.
The production of fluoroantimonic acid involves the use of hydrofluoric acid and antimony pentafluoride, both of which have their own environmental implications. The manufacturing process may contribute to air pollution through the release of fluorine-containing compounds, which are known to have negative impacts on air quality and can contribute to the depletion of the ozone layer.
In terms of waste management, the disposal of batteries containing fluoroantimonic acid presents significant challenges. Conventional recycling methods may not be suitable due to the acid's extreme corrosiveness, potentially leading to increased hazardous waste generation. This could strain existing waste management infrastructure and increase the risk of environmental contamination.
The long-term environmental effects of fluoroantimonic acid exposure are not yet fully understood, but studies suggest potential bioaccumulation in food chains and persistent environmental contamination. This raises concerns about its impact on biodiversity and ecosystem stability over extended periods.
From a lifecycle perspective, the environmental footprint of fluoroantimonic acid-based batteries extends beyond their use phase. The extraction and processing of raw materials required for acid production, such as antimony and fluorine, can have substantial environmental impacts, including habitat destruction and resource depletion.
To mitigate these environmental risks, stringent safety protocols and containment measures would be necessary throughout the battery lifecycle. This includes robust packaging for transportation, secure storage facilities, and specialized handling procedures during manufacturing and recycling processes. Additionally, the development of effective neutralization and remediation techniques would be crucial for addressing potential spills or contamination incidents.
As the battery industry continues to explore innovative technologies, the environmental implications of fluoroantimonic acid must be carefully weighed against its potential benefits. Sustainable alternatives and improved safety measures should be actively researched to minimize the ecological footprint of this powerful but potentially hazardous substance in battery applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!