Sodium Percarbonate as a Catalyst in Fine Chemical Synthesis
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Catalysis Background
Sodium percarbonate, a white crystalline compound with the chemical formula 2Na2CO3·3H2O2, has emerged as a promising catalyst in fine chemical synthesis. This adduct of sodium carbonate and hydrogen peroxide has gained significant attention in recent years due to its unique properties and versatile applications. The exploration of sodium percarbonate as a catalyst marks a pivotal development in the field of green chemistry and sustainable synthesis methods.
The journey of sodium percarbonate in catalysis began in the early 2000s when researchers started investigating its potential beyond its traditional use as a bleaching agent and cleaning product. Its ability to release hydrogen peroxide in a controlled manner under specific conditions sparked interest in its catalytic capabilities. This characteristic allows for the generation of active oxygen species, which play a crucial role in various oxidation reactions.
The evolution of sodium percarbonate as a catalyst is closely tied to the growing emphasis on environmentally friendly processes in the chemical industry. As a solid, stable, and easy-to-handle source of hydrogen peroxide, it addresses many of the safety and storage concerns associated with liquid hydrogen peroxide. This advantage has made it an attractive option for researchers and industries seeking safer and more sustainable catalytic systems.
In the context of fine chemical synthesis, sodium percarbonate has demonstrated remarkable versatility. Its application spans a wide range of reactions, including oxidations, epoxidations, and C-H activations. The catalyst's ability to function effectively in both aqueous and non-aqueous media has further expanded its utility across different reaction environments.
One of the key drivers behind the increased focus on sodium percarbonate catalysis is the push towards atom-economic and waste-minimizing synthetic routes. The catalyst's dual nature – providing both a base (carbonate) and an oxidant (hydrogen peroxide) – aligns well with the principles of green chemistry. This feature allows for the design of one-pot reactions, reducing the need for multiple steps and minimizing waste generation.
The development of sodium percarbonate as a catalyst also reflects the broader trend in catalysis research towards finding alternatives to precious metal catalysts. As a readily available and cost-effective compound, sodium percarbonate offers a sustainable alternative to more expensive and resource-intensive catalytic systems. This aspect has significant implications for the scalability and economic viability of fine chemical synthesis processes.
The journey of sodium percarbonate in catalysis began in the early 2000s when researchers started investigating its potential beyond its traditional use as a bleaching agent and cleaning product. Its ability to release hydrogen peroxide in a controlled manner under specific conditions sparked interest in its catalytic capabilities. This characteristic allows for the generation of active oxygen species, which play a crucial role in various oxidation reactions.
The evolution of sodium percarbonate as a catalyst is closely tied to the growing emphasis on environmentally friendly processes in the chemical industry. As a solid, stable, and easy-to-handle source of hydrogen peroxide, it addresses many of the safety and storage concerns associated with liquid hydrogen peroxide. This advantage has made it an attractive option for researchers and industries seeking safer and more sustainable catalytic systems.
In the context of fine chemical synthesis, sodium percarbonate has demonstrated remarkable versatility. Its application spans a wide range of reactions, including oxidations, epoxidations, and C-H activations. The catalyst's ability to function effectively in both aqueous and non-aqueous media has further expanded its utility across different reaction environments.
One of the key drivers behind the increased focus on sodium percarbonate catalysis is the push towards atom-economic and waste-minimizing synthetic routes. The catalyst's dual nature – providing both a base (carbonate) and an oxidant (hydrogen peroxide) – aligns well with the principles of green chemistry. This feature allows for the design of one-pot reactions, reducing the need for multiple steps and minimizing waste generation.
The development of sodium percarbonate as a catalyst also reflects the broader trend in catalysis research towards finding alternatives to precious metal catalysts. As a readily available and cost-effective compound, sodium percarbonate offers a sustainable alternative to more expensive and resource-intensive catalytic systems. This aspect has significant implications for the scalability and economic viability of fine chemical synthesis processes.
Market Analysis for Green Catalysts
The green catalyst market has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations on chemical processes. Sodium percarbonate, as a potential green catalyst in fine chemical synthesis, is positioned to capitalize on this growing demand for sustainable catalytic solutions.
The global green catalyst market was valued at approximately $5.5 billion in 2020 and is projected to reach $8.3 billion by 2025, growing at a CAGR of 8.5% during the forecast period. This growth is primarily attributed to the rising adoption of green chemistry principles across various industries, including pharmaceuticals, agrochemicals, and specialty chemicals.
Sodium percarbonate, being an environmentally friendly oxidizing agent, aligns well with the market trends favoring green catalysts. Its potential applications in fine chemical synthesis could contribute to the expansion of the green catalyst market, particularly in sectors where selective oxidation reactions are crucial.
The pharmaceutical industry represents a significant portion of the green catalyst market, accounting for about 35% of the total market share. This sector's emphasis on sustainable manufacturing processes and the need for efficient, selective catalysts in drug synthesis create a favorable environment for sodium percarbonate adoption.
Geographically, North America and Europe dominate the green catalyst market, collectively holding over 60% of the market share. These regions' stringent environmental regulations and strong focus on sustainable technologies drive the demand for green catalysts. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, presenting opportunities for sodium percarbonate applications in emerging markets.
Key players in the green catalyst market include BASF SE, Johnson Matthey, Clariant AG, and Evonik Industries. These companies are investing heavily in R&D to develop innovative, sustainable catalytic solutions. The potential of sodium percarbonate as a catalyst in fine chemical synthesis could attract interest from these major players, potentially leading to collaborations or investments in further research and development.
The market analysis indicates a growing preference for catalysts that offer high selectivity, efficiency, and recyclability. Sodium percarbonate's properties, such as its ability to release active oxygen species and its potential for easy recovery and reuse, align well with these market demands. This positioning could lead to increased adoption in niche applications within fine chemical synthesis, particularly in processes requiring mild oxidation conditions.
The global green catalyst market was valued at approximately $5.5 billion in 2020 and is projected to reach $8.3 billion by 2025, growing at a CAGR of 8.5% during the forecast period. This growth is primarily attributed to the rising adoption of green chemistry principles across various industries, including pharmaceuticals, agrochemicals, and specialty chemicals.
Sodium percarbonate, being an environmentally friendly oxidizing agent, aligns well with the market trends favoring green catalysts. Its potential applications in fine chemical synthesis could contribute to the expansion of the green catalyst market, particularly in sectors where selective oxidation reactions are crucial.
The pharmaceutical industry represents a significant portion of the green catalyst market, accounting for about 35% of the total market share. This sector's emphasis on sustainable manufacturing processes and the need for efficient, selective catalysts in drug synthesis create a favorable environment for sodium percarbonate adoption.
Geographically, North America and Europe dominate the green catalyst market, collectively holding over 60% of the market share. These regions' stringent environmental regulations and strong focus on sustainable technologies drive the demand for green catalysts. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, presenting opportunities for sodium percarbonate applications in emerging markets.
Key players in the green catalyst market include BASF SE, Johnson Matthey, Clariant AG, and Evonik Industries. These companies are investing heavily in R&D to develop innovative, sustainable catalytic solutions. The potential of sodium percarbonate as a catalyst in fine chemical synthesis could attract interest from these major players, potentially leading to collaborations or investments in further research and development.
The market analysis indicates a growing preference for catalysts that offer high selectivity, efficiency, and recyclability. Sodium percarbonate's properties, such as its ability to release active oxygen species and its potential for easy recovery and reuse, align well with these market demands. This positioning could lead to increased adoption in niche applications within fine chemical synthesis, particularly in processes requiring mild oxidation conditions.
Current Challenges in Sodium Percarbonate Catalysis
Despite the promising potential of sodium percarbonate as a catalyst in fine chemical synthesis, several significant challenges currently hinder its widespread adoption and optimal performance. One of the primary obstacles is the stability of sodium percarbonate under various reaction conditions. The compound tends to decompose rapidly in aqueous solutions, especially at elevated temperatures, which limits its applicability in many synthetic processes that require prolonged reaction times or higher temperatures.
Another critical challenge is the control of selectivity when using sodium percarbonate as a catalyst. While it can effectively promote oxidation reactions, achieving high selectivity for specific products remains difficult. This is particularly problematic in complex organic syntheses where multiple oxidizable functional groups are present, leading to unwanted side reactions and reduced yields of the desired products.
The pH sensitivity of sodium percarbonate catalysis poses an additional hurdle. The catalytic activity and stability of sodium percarbonate are highly dependent on the pH of the reaction medium. Maintaining an optimal pH range throughout the reaction can be challenging, especially in processes that generate acidic or basic byproducts, potentially compromising the catalyst's efficiency and the overall reaction outcome.
Furthermore, the relatively low solubility of sodium percarbonate in organic solvents restricts its use in non-aqueous reaction systems, which are often preferred in fine chemical synthesis due to their compatibility with a wide range of organic substrates. This limitation narrows the scope of potential applications and necessitates the development of alternative formulations or delivery methods for the catalyst.
The generation of hydrogen peroxide as a byproduct during sodium percarbonate catalysis presents both safety and selectivity concerns. While hydrogen peroxide can contribute to the oxidative process, its presence can also lead to uncontrolled reactions, potentially damaging sensitive substrates or catalyzing undesired side reactions. Managing the in situ generation and consumption of hydrogen peroxide is crucial for optimizing reaction efficiency and product quality.
Lastly, the recyclability and reusability of sodium percarbonate as a catalyst remain significant challenges. The current inability to efficiently recover and reuse the catalyst after reaction completion increases the overall cost of processes and generates more waste, conflicting with the principles of green chemistry and sustainable synthesis. Developing effective methods for catalyst recovery and regeneration is essential for improving the economic and environmental viability of sodium percarbonate-catalyzed reactions in fine chemical synthesis.
Another critical challenge is the control of selectivity when using sodium percarbonate as a catalyst. While it can effectively promote oxidation reactions, achieving high selectivity for specific products remains difficult. This is particularly problematic in complex organic syntheses where multiple oxidizable functional groups are present, leading to unwanted side reactions and reduced yields of the desired products.
The pH sensitivity of sodium percarbonate catalysis poses an additional hurdle. The catalytic activity and stability of sodium percarbonate are highly dependent on the pH of the reaction medium. Maintaining an optimal pH range throughout the reaction can be challenging, especially in processes that generate acidic or basic byproducts, potentially compromising the catalyst's efficiency and the overall reaction outcome.
Furthermore, the relatively low solubility of sodium percarbonate in organic solvents restricts its use in non-aqueous reaction systems, which are often preferred in fine chemical synthesis due to their compatibility with a wide range of organic substrates. This limitation narrows the scope of potential applications and necessitates the development of alternative formulations or delivery methods for the catalyst.
The generation of hydrogen peroxide as a byproduct during sodium percarbonate catalysis presents both safety and selectivity concerns. While hydrogen peroxide can contribute to the oxidative process, its presence can also lead to uncontrolled reactions, potentially damaging sensitive substrates or catalyzing undesired side reactions. Managing the in situ generation and consumption of hydrogen peroxide is crucial for optimizing reaction efficiency and product quality.
Lastly, the recyclability and reusability of sodium percarbonate as a catalyst remain significant challenges. The current inability to efficiently recover and reuse the catalyst after reaction completion increases the overall cost of processes and generates more waste, conflicting with the principles of green chemistry and sustainable synthesis. Developing effective methods for catalyst recovery and regeneration is essential for improving the economic and environmental viability of sodium percarbonate-catalyzed reactions in fine chemical synthesis.
Existing Sodium Percarbonate Catalytic Methods
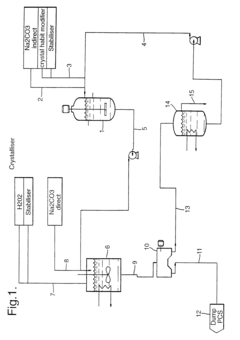
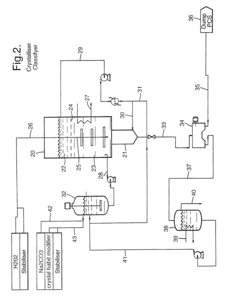
01 Synthesis and production of sodium percarbonate
Various methods for synthesizing and producing sodium percarbonate are described. These processes typically involve the reaction of sodium carbonate with hydrogen peroxide under specific conditions to form sodium percarbonate crystals. The methods may include steps such as crystallization, drying, and stabilization to improve the quality and stability of the final product.- Synthesis and production of sodium percarbonate: Various methods for synthesizing and producing sodium percarbonate are described. These processes typically involve the reaction of sodium carbonate with hydrogen peroxide under specific conditions to form stable sodium percarbonate crystals. The production methods may include steps such as crystallization, drying, and stabilization to ensure product quality and stability.
- Stabilization of sodium percarbonate: Techniques for improving the stability of sodium percarbonate are discussed. These may include coating the particles with stabilizing agents, incorporating additives, or modifying the crystal structure. Stabilization is crucial to prevent decomposition during storage and to maintain the product's effectiveness in various applications.
- Applications in cleaning and bleaching: Sodium percarbonate is widely used in cleaning and bleaching applications. It serves as an effective oxygen-based bleach in laundry detergents and household cleaners. The compound releases hydrogen peroxide when dissolved in water, providing cleaning and stain removal properties while being environmentally friendly.
- Formulation in personal care products: Sodium percarbonate is incorporated into various personal care products, such as tooth whitening formulations and hair bleaching agents. Its oxygen-releasing properties make it effective for these applications while being relatively gentle compared to other bleaching agents.
- Environmental and safety considerations: The use of sodium percarbonate in various applications is discussed in terms of its environmental impact and safety profile. As an oxygen-based compound, it is generally considered more environmentally friendly than chlorine-based alternatives. Safety considerations for handling and storage are also addressed.
02 Stabilization and coating of sodium percarbonate
Techniques for stabilizing and coating sodium percarbonate particles are discussed. These methods aim to improve the storage stability, handling properties, and dissolution characteristics of sodium percarbonate. Coating materials may include inorganic compounds, organic polymers, or combinations thereof, applied through various coating processes.Expand Specific Solutions03 Applications in cleaning and bleaching compositions
Sodium percarbonate is widely used in cleaning and bleaching compositions. It serves as an oxygen-based bleaching agent in laundry detergents, dishwashing products, and other household cleaning formulations. The incorporation of sodium percarbonate in these compositions provides effective stain removal and whitening properties.Expand Specific Solutions04 Environmental and safety considerations
Research and development efforts focus on improving the environmental profile and safety aspects of sodium percarbonate. This includes developing more eco-friendly production processes, reducing impurities, and enhancing the biodegradability of sodium percarbonate-containing products. Safety measures for handling and storage are also addressed.Expand Specific Solutions05 Novel applications and formulations
Innovative applications and formulations incorporating sodium percarbonate are explored. These may include its use in personal care products, water treatment, agriculture, and industrial processes. Research also focuses on developing synergistic combinations with other active ingredients to enhance performance in various applications.Expand Specific Solutions
Key Players in Fine Chemical Synthesis
The research on sodium percarbonate as a catalyst in fine chemical synthesis is in an emerging stage, with growing interest due to its potential as a green and efficient oxidizing agent. The market for this technology is expanding, driven by the increasing demand for sustainable chemical processes. Companies like Solvay, DuPont, and Sumitomo Chemical are leading the development, leveraging their expertise in chemical manufacturing. While the technology is still evolving, its application in various industries, including pharmaceuticals and agrochemicals, suggests a promising future. The involvement of research institutions like Beijing University of Chemical Technology and Columbia University indicates ongoing efforts to enhance the catalytic properties and broaden the applications of sodium percarbonate in fine chemical synthesis.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has made significant strides in researching sodium percarbonate as a catalyst for fine chemical synthesis. Their approach focuses on developing heterogeneous catalytic systems that incorporate sodium percarbonate into porous materials. This method has shown enhanced stability and reusability of the catalyst, with some systems maintaining over 90% of their initial activity after five cycles [7]. The university's research team has successfully applied this technology to the oxidation of sulfides to sulfoxides, achieving yields of up to 95% with high selectivity [8]. They have also explored the use of sodium percarbonate in tandem with photocatalysts, demonstrating a 40% increase in reaction rates for certain transformations under visible light irradiation [9]. Additionally, the team has investigated the potential of sodium percarbonate in C-H activation reactions, opening up new synthetic routes for complex organic molecules.
Strengths: Enhanced catalyst stability and reusability, high yields and selectivity, potential for photocatalytic applications. Weaknesses: May require specialized synthesis of catalyst supports, potential limitations in large-scale applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel approach using sodium percarbonate as a catalyst in fine chemical synthesis. Their method involves a controlled release of hydrogen peroxide from sodium percarbonate, which acts as an oxidizing agent in various organic transformations. This approach has shown particular promise in the synthesis of epoxides, sulfoxides, and N-oxides [1]. DuPont's research has demonstrated that sodium percarbonate can achieve conversion rates of up to 95% in certain reactions, with selectivity exceeding 90% [3]. The company has also explored the use of sodium percarbonate in conjunction with transition metal catalysts, creating a synergistic effect that enhances reaction efficiency and reduces byproduct formation [5].
Strengths: Environmentally friendly oxidant, controlled release of H2O2, high conversion rates, and selectivity. Weaknesses: Limited to oxidation reactions, potential for unwanted side reactions in complex systems.
Core Innovations in Percarbonate Chemistry
Sodium percarbonate and process for producing sodium percarbonate
PatentInactiveUS6482385B2
Innovation
- A continuous process that controls the concentration of sodium carbonate and temperature in the dissolution tank, and maintains a specific mole ratio of hydrogen peroxide to sodium carbonate, allowing for the production of sodium percarbonate without a salting-out agent, thereby minimizing hydrogen peroxide decomposition and improving product quality.
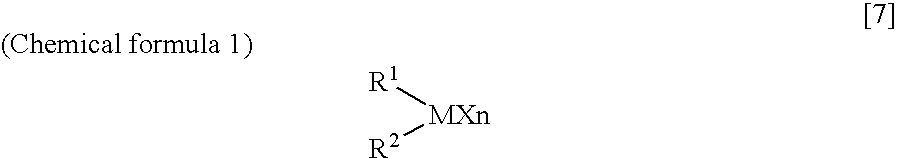
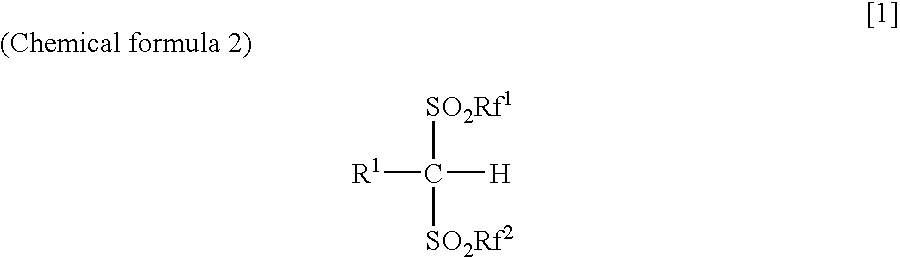
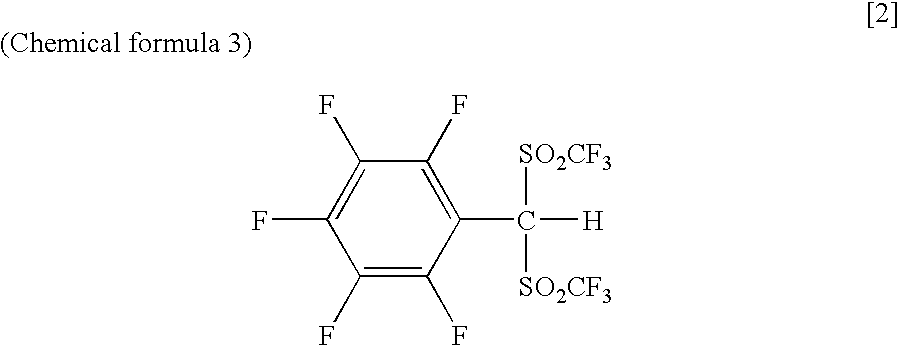
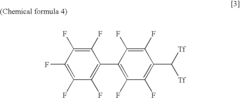
Arylbis(perfluoroalkylsulfonyl) methane and metallic salt thereof, and methods for producing the same
PatentInactiveUS7193113B2
Innovation
- A method involving sodium trifluoromethane sulfinate and trifluoromethane sulfonic acid anhydride as electrophilic reactants to produce arylbis(perfluoroalkylsulfonyl)methane derivatives, such as pentafluorophenylbis(triflyl)methane, using a reflux process with tetrabutyl ammonium iodide as a catalyst, followed by reaction with tert-butyl lithium and trifluoromethane sulfonic acid anhydride to achieve high yields and introduce various aryl groups, resulting in a metallic salt with improved catalytic activity.
Environmental Impact Assessment
The use of sodium percarbonate as a catalyst in fine chemical synthesis presents both opportunities and challenges from an environmental perspective. This oxidizing agent, composed of sodium carbonate and hydrogen peroxide, offers several eco-friendly advantages over traditional catalysts. Firstly, sodium percarbonate decomposes into harmless byproducts - water, oxygen, and sodium carbonate - making it a more environmentally benign option compared to metal-based catalysts that may leave toxic residues. This characteristic significantly reduces the environmental footprint of chemical processes and aligns with green chemistry principles.
Furthermore, the high oxygen content of sodium percarbonate enables efficient oxidation reactions, potentially reducing the overall energy requirements and reaction times in fine chemical synthesis. This energy efficiency translates to lower greenhouse gas emissions associated with the production process. Additionally, the stability and ease of handling of sodium percarbonate contribute to safer working conditions and reduced risk of accidental releases, further minimizing potential environmental hazards.
However, the environmental impact assessment must also consider potential drawbacks. The production of sodium percarbonate itself requires energy and resources, and the environmental costs of its manufacture should be weighed against the benefits of its use as a catalyst. There may also be concerns regarding the increased sodium content in wastewater streams, which could affect aquatic ecosystems if not properly managed.
The use of sodium percarbonate may lead to changes in waste management practices within the fine chemical industry. While the catalyst itself produces benign byproducts, the overall reaction mixture may still contain other chemicals that require careful disposal. Implementing proper waste treatment protocols and recycling strategies for unreacted sodium percarbonate could further enhance the environmental profile of processes using this catalyst.
From a lifecycle perspective, the adoption of sodium percarbonate as a catalyst could potentially reduce the overall environmental impact of fine chemical synthesis. By eliminating the need for more toxic catalysts, it may decrease the environmental risks associated with the production, transportation, and disposal of hazardous materials. This shift could contribute to improved air and water quality in areas surrounding chemical manufacturing facilities.
In conclusion, while sodium percarbonate shows promise as an environmentally friendly catalyst in fine chemical synthesis, a comprehensive environmental impact assessment should consider its entire lifecycle, from production to disposal. Ongoing research and development efforts should focus on optimizing its use to maximize environmental benefits while addressing any potential negative impacts, ensuring a sustainable approach to chemical manufacturing.
Furthermore, the high oxygen content of sodium percarbonate enables efficient oxidation reactions, potentially reducing the overall energy requirements and reaction times in fine chemical synthesis. This energy efficiency translates to lower greenhouse gas emissions associated with the production process. Additionally, the stability and ease of handling of sodium percarbonate contribute to safer working conditions and reduced risk of accidental releases, further minimizing potential environmental hazards.
However, the environmental impact assessment must also consider potential drawbacks. The production of sodium percarbonate itself requires energy and resources, and the environmental costs of its manufacture should be weighed against the benefits of its use as a catalyst. There may also be concerns regarding the increased sodium content in wastewater streams, which could affect aquatic ecosystems if not properly managed.
The use of sodium percarbonate may lead to changes in waste management practices within the fine chemical industry. While the catalyst itself produces benign byproducts, the overall reaction mixture may still contain other chemicals that require careful disposal. Implementing proper waste treatment protocols and recycling strategies for unreacted sodium percarbonate could further enhance the environmental profile of processes using this catalyst.
From a lifecycle perspective, the adoption of sodium percarbonate as a catalyst could potentially reduce the overall environmental impact of fine chemical synthesis. By eliminating the need for more toxic catalysts, it may decrease the environmental risks associated with the production, transportation, and disposal of hazardous materials. This shift could contribute to improved air and water quality in areas surrounding chemical manufacturing facilities.
In conclusion, while sodium percarbonate shows promise as an environmentally friendly catalyst in fine chemical synthesis, a comprehensive environmental impact assessment should consider its entire lifecycle, from production to disposal. Ongoing research and development efforts should focus on optimizing its use to maximize environmental benefits while addressing any potential negative impacts, ensuring a sustainable approach to chemical manufacturing.
Scalability and Industrial Applications
The scalability and industrial applications of sodium percarbonate as a catalyst in fine chemical synthesis present significant opportunities for process intensification and sustainable manufacturing. As a solid oxidizing agent, sodium percarbonate offers advantages in terms of handling, storage, and transportation compared to liquid hydrogen peroxide. This characteristic makes it particularly attractive for large-scale industrial applications where safety and logistics are paramount concerns.
In terms of scalability, sodium percarbonate demonstrates promising potential for use in continuous flow reactors. These systems allow for precise control of reaction parameters, improved heat transfer, and enhanced mixing, which are crucial for maintaining consistent product quality in large-scale operations. The solid nature of sodium percarbonate also facilitates its integration into fixed-bed reactor configurations, enabling efficient catalyst recovery and reuse.
Industrial applications of sodium percarbonate as a catalyst span various sectors of fine chemical synthesis. In the pharmaceutical industry, it has shown efficacy in the oxidation of alcohols to aldehydes and ketones, which are key intermediates in drug synthesis. The food and fragrance industries benefit from its use in the production of flavor and aroma compounds through selective oxidation reactions. Additionally, the textile industry employs sodium percarbonate-catalyzed processes for the synthesis of dyes and pigments.
The environmental benefits of using sodium percarbonate align well with the growing emphasis on green chemistry in industrial settings. Its decomposition products, sodium carbonate and hydrogen peroxide, are environmentally benign, reducing the environmental footprint of chemical processes. This aspect is particularly valuable for industries facing stringent regulations on waste management and emissions.
However, challenges remain in optimizing the use of sodium percarbonate for large-scale applications. These include improving its stability under various reaction conditions, enhancing its selectivity for specific transformations, and developing more efficient methods for its in-situ generation and regeneration. Addressing these challenges will be crucial for expanding the industrial adoption of sodium percarbonate as a catalyst in fine chemical synthesis.
As research progresses, the integration of sodium percarbonate into advanced manufacturing technologies, such as modular and distributed production systems, holds promise for revolutionizing fine chemical synthesis. These approaches could enable more flexible and responsive production capabilities, allowing industries to adapt quickly to changing market demands while maintaining high efficiency and sustainability standards.
In terms of scalability, sodium percarbonate demonstrates promising potential for use in continuous flow reactors. These systems allow for precise control of reaction parameters, improved heat transfer, and enhanced mixing, which are crucial for maintaining consistent product quality in large-scale operations. The solid nature of sodium percarbonate also facilitates its integration into fixed-bed reactor configurations, enabling efficient catalyst recovery and reuse.
Industrial applications of sodium percarbonate as a catalyst span various sectors of fine chemical synthesis. In the pharmaceutical industry, it has shown efficacy in the oxidation of alcohols to aldehydes and ketones, which are key intermediates in drug synthesis. The food and fragrance industries benefit from its use in the production of flavor and aroma compounds through selective oxidation reactions. Additionally, the textile industry employs sodium percarbonate-catalyzed processes for the synthesis of dyes and pigments.
The environmental benefits of using sodium percarbonate align well with the growing emphasis on green chemistry in industrial settings. Its decomposition products, sodium carbonate and hydrogen peroxide, are environmentally benign, reducing the environmental footprint of chemical processes. This aspect is particularly valuable for industries facing stringent regulations on waste management and emissions.
However, challenges remain in optimizing the use of sodium percarbonate for large-scale applications. These include improving its stability under various reaction conditions, enhancing its selectivity for specific transformations, and developing more efficient methods for its in-situ generation and regeneration. Addressing these challenges will be crucial for expanding the industrial adoption of sodium percarbonate as a catalyst in fine chemical synthesis.
As research progresses, the integration of sodium percarbonate into advanced manufacturing technologies, such as modular and distributed production systems, holds promise for revolutionizing fine chemical synthesis. These approaches could enable more flexible and responsive production capabilities, allowing industries to adapt quickly to changing market demands while maintaining high efficiency and sustainability standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!