Silicon photonics and its impact on wearable medical devices.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicon Photonics Evolution and Objectives
Silicon photonics has emerged as a transformative technology in the field of integrated optics, with its roots tracing back to the late 1980s. The evolution of this technology has been driven by the increasing demand for high-speed data transmission and processing in various applications, including telecommunications, data centers, and more recently, wearable medical devices.
The initial development of silicon photonics focused on leveraging the existing silicon manufacturing infrastructure to create optical components on a chip. This approach aimed to overcome the limitations of traditional electronic circuits in terms of speed and power consumption. As the technology progressed, researchers and engineers made significant strides in developing key components such as waveguides, modulators, and photodetectors on silicon substrates.
In the context of wearable medical devices, the objectives of silicon photonics have expanded beyond data transmission to include sensing and diagnostic capabilities. The integration of optical components with electronic circuits on a single chip has opened up new possibilities for miniaturization and enhanced functionality in wearable health monitoring systems.
One of the primary goals of silicon photonics in wearable medical devices is to enable real-time, non-invasive monitoring of various physiological parameters. This includes measuring blood glucose levels, oxygen saturation, heart rate, and even detecting specific biomarkers in bodily fluids. The ability to perform these measurements with high accuracy and reliability is crucial for the widespread adoption of wearable medical devices.
Another objective is to improve the power efficiency of these devices, allowing for longer battery life and more comfortable wear. Silicon photonics offers the potential for low-power operation due to its ability to manipulate light signals with minimal energy consumption. This is particularly important for continuous health monitoring applications where device longevity is critical.
Furthermore, the integration of silicon photonics in wearable medical devices aims to enhance data processing capabilities at the edge. By incorporating on-chip optical computing elements, these devices can perform complex analysis of health data in real-time, reducing the need for constant communication with external systems and improving response times in critical situations.
As the field continues to advance, researchers are exploring novel materials and structures to overcome current limitations and push the boundaries of what is possible with silicon photonics. This includes the development of hybrid integration techniques that combine silicon with other materials to achieve enhanced functionality and performance in wearable medical applications.
The initial development of silicon photonics focused on leveraging the existing silicon manufacturing infrastructure to create optical components on a chip. This approach aimed to overcome the limitations of traditional electronic circuits in terms of speed and power consumption. As the technology progressed, researchers and engineers made significant strides in developing key components such as waveguides, modulators, and photodetectors on silicon substrates.
In the context of wearable medical devices, the objectives of silicon photonics have expanded beyond data transmission to include sensing and diagnostic capabilities. The integration of optical components with electronic circuits on a single chip has opened up new possibilities for miniaturization and enhanced functionality in wearable health monitoring systems.
One of the primary goals of silicon photonics in wearable medical devices is to enable real-time, non-invasive monitoring of various physiological parameters. This includes measuring blood glucose levels, oxygen saturation, heart rate, and even detecting specific biomarkers in bodily fluids. The ability to perform these measurements with high accuracy and reliability is crucial for the widespread adoption of wearable medical devices.
Another objective is to improve the power efficiency of these devices, allowing for longer battery life and more comfortable wear. Silicon photonics offers the potential for low-power operation due to its ability to manipulate light signals with minimal energy consumption. This is particularly important for continuous health monitoring applications where device longevity is critical.
Furthermore, the integration of silicon photonics in wearable medical devices aims to enhance data processing capabilities at the edge. By incorporating on-chip optical computing elements, these devices can perform complex analysis of health data in real-time, reducing the need for constant communication with external systems and improving response times in critical situations.
As the field continues to advance, researchers are exploring novel materials and structures to overcome current limitations and push the boundaries of what is possible with silicon photonics. This includes the development of hybrid integration techniques that combine silicon with other materials to achieve enhanced functionality and performance in wearable medical applications.
Wearable Medical Device Market Analysis
The wearable medical device market has experienced significant growth in recent years, driven by advancements in technology, increasing health awareness, and the growing prevalence of chronic diseases. This market segment encompasses a wide range of devices, including fitness trackers, smartwatches with health monitoring capabilities, continuous glucose monitors, and wearable ECG monitors.
The global wearable medical device market is expected to continue its upward trajectory, with projections indicating substantial growth over the next decade. This expansion is fueled by several factors, including the aging population, rising healthcare costs, and the shift towards preventive healthcare and remote patient monitoring.
One of the key drivers of market growth is the increasing adoption of wearable devices for chronic disease management. Conditions such as diabetes, cardiovascular diseases, and respiratory disorders require continuous monitoring, which can be effectively achieved through wearable medical devices. These devices offer real-time data collection and analysis, enabling healthcare providers to make timely interventions and adjust treatment plans as needed.
The integration of advanced technologies, such as artificial intelligence and machine learning, into wearable medical devices is enhancing their capabilities and expanding their potential applications. These technologies enable more accurate data interpretation, personalized health insights, and predictive analytics, further driving market growth and innovation.
The COVID-19 pandemic has also accelerated the adoption of wearable medical devices, as healthcare systems worldwide sought ways to monitor patients remotely and reduce the risk of virus transmission. This trend is expected to continue even after the pandemic, as both patients and healthcare providers recognize the benefits of remote monitoring and telemedicine.
Geographically, North America currently holds the largest share of the wearable medical device market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing disposable income, and growing awareness of personal health management.
Despite the positive outlook, the wearable medical device market faces challenges such as data privacy concerns, regulatory hurdles, and the need for improved accuracy and reliability of devices. Addressing these issues will be crucial for sustained market growth and wider adoption of wearable medical technologies.
The global wearable medical device market is expected to continue its upward trajectory, with projections indicating substantial growth over the next decade. This expansion is fueled by several factors, including the aging population, rising healthcare costs, and the shift towards preventive healthcare and remote patient monitoring.
One of the key drivers of market growth is the increasing adoption of wearable devices for chronic disease management. Conditions such as diabetes, cardiovascular diseases, and respiratory disorders require continuous monitoring, which can be effectively achieved through wearable medical devices. These devices offer real-time data collection and analysis, enabling healthcare providers to make timely interventions and adjust treatment plans as needed.
The integration of advanced technologies, such as artificial intelligence and machine learning, into wearable medical devices is enhancing their capabilities and expanding their potential applications. These technologies enable more accurate data interpretation, personalized health insights, and predictive analytics, further driving market growth and innovation.
The COVID-19 pandemic has also accelerated the adoption of wearable medical devices, as healthcare systems worldwide sought ways to monitor patients remotely and reduce the risk of virus transmission. This trend is expected to continue even after the pandemic, as both patients and healthcare providers recognize the benefits of remote monitoring and telemedicine.
Geographically, North America currently holds the largest share of the wearable medical device market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing disposable income, and growing awareness of personal health management.
Despite the positive outlook, the wearable medical device market faces challenges such as data privacy concerns, regulatory hurdles, and the need for improved accuracy and reliability of devices. Addressing these issues will be crucial for sustained market growth and wider adoption of wearable medical technologies.
Silicon Photonics: Current State and Challenges
Silicon photonics has emerged as a transformative technology in the field of integrated optics, offering unprecedented opportunities for miniaturization, energy efficiency, and high-speed data transmission. The current state of silicon photonics is characterized by significant advancements in device fabrication, integration techniques, and system-level implementations. However, several challenges remain to be addressed for its widespread adoption in wearable medical devices.
One of the primary achievements in silicon photonics is the development of high-performance optical components on a silicon platform. These include low-loss waveguides, efficient modulators, and sensitive photodetectors. The ability to integrate these components with CMOS electronics has paved the way for compact, power-efficient photonic integrated circuits (PICs). This integration capability is particularly crucial for wearable medical devices, where size and power consumption are critical factors.
Despite these advancements, silicon photonics faces several technical challenges. One significant hurdle is the efficient coupling of light between optical fibers and silicon waveguides. The mode size mismatch and alignment requirements pose difficulties in achieving low-loss coupling, which is essential for maintaining signal integrity in wearable medical devices. Various approaches, such as grating couplers and edge couplers, have been developed to address this issue, but further improvements in coupling efficiency and bandwidth are still needed.
Another challenge lies in the development of efficient light sources integrated on silicon. Silicon's indirect bandgap makes it an inefficient light emitter, necessitating the integration of III-V materials or the exploration of alternative light generation mechanisms. While hybrid integration techniques have shown promise, achieving reliable and cost-effective integration of light sources remains a significant challenge for silicon photonics in wearable medical applications.
Temperature sensitivity is another critical issue in silicon photonic devices. The thermo-optic effect in silicon can cause wavelength shifts and performance degradation in photonic components. This is particularly problematic for wearable medical devices that may be subject to varying environmental conditions. Developing robust temperature compensation techniques and thermally stable designs is crucial for ensuring reliable operation in real-world scenarios.
Furthermore, the scalability and manufacturability of silicon photonic devices present ongoing challenges. While silicon photonics leverages existing CMOS fabrication infrastructure, achieving high yields and consistent performance across large-scale production remains difficult. This is particularly important for wearable medical devices, where reliability and cost-effectiveness are paramount.
In the context of wearable medical devices, additional challenges arise from the need for biocompatibility, flexibility, and long-term stability. Integrating silicon photonic components into flexible substrates and ensuring their durability under repeated mechanical stress are areas that require further research and development.
One of the primary achievements in silicon photonics is the development of high-performance optical components on a silicon platform. These include low-loss waveguides, efficient modulators, and sensitive photodetectors. The ability to integrate these components with CMOS electronics has paved the way for compact, power-efficient photonic integrated circuits (PICs). This integration capability is particularly crucial for wearable medical devices, where size and power consumption are critical factors.
Despite these advancements, silicon photonics faces several technical challenges. One significant hurdle is the efficient coupling of light between optical fibers and silicon waveguides. The mode size mismatch and alignment requirements pose difficulties in achieving low-loss coupling, which is essential for maintaining signal integrity in wearable medical devices. Various approaches, such as grating couplers and edge couplers, have been developed to address this issue, but further improvements in coupling efficiency and bandwidth are still needed.
Another challenge lies in the development of efficient light sources integrated on silicon. Silicon's indirect bandgap makes it an inefficient light emitter, necessitating the integration of III-V materials or the exploration of alternative light generation mechanisms. While hybrid integration techniques have shown promise, achieving reliable and cost-effective integration of light sources remains a significant challenge for silicon photonics in wearable medical applications.
Temperature sensitivity is another critical issue in silicon photonic devices. The thermo-optic effect in silicon can cause wavelength shifts and performance degradation in photonic components. This is particularly problematic for wearable medical devices that may be subject to varying environmental conditions. Developing robust temperature compensation techniques and thermally stable designs is crucial for ensuring reliable operation in real-world scenarios.
Furthermore, the scalability and manufacturability of silicon photonic devices present ongoing challenges. While silicon photonics leverages existing CMOS fabrication infrastructure, achieving high yields and consistent performance across large-scale production remains difficult. This is particularly important for wearable medical devices, where reliability and cost-effectiveness are paramount.
In the context of wearable medical devices, additional challenges arise from the need for biocompatibility, flexibility, and long-term stability. Integrating silicon photonic components into flexible substrates and ensuring their durability under repeated mechanical stress are areas that require further research and development.
Silicon Photonics Integration Solutions
01 Integrated photonic devices
Silicon photonics technology enables the integration of various optical components on a single chip. This includes waveguides, modulators, detectors, and other photonic elements, allowing for compact and efficient optical systems. The integration of these components facilitates high-speed data transmission and processing in optical communication networks and computing applications.- Integration of optical components on silicon: Silicon photonics involves integrating optical components onto silicon substrates, enabling the creation of high-performance photonic devices. This technology allows for the fabrication of optical waveguides, modulators, and detectors on a single chip, facilitating the development of compact and efficient optical communication systems.
- Optical interconnects for data transmission: Silicon photonics enables the development of optical interconnects for high-speed data transmission. These interconnects use light to transmit data between chips or within a chip, offering advantages such as higher bandwidth, lower power consumption, and reduced latency compared to traditional electrical interconnects.
- Integration with electronic circuits: Silicon photonics allows for the integration of optical components with electronic circuits on a single chip. This integration enables the development of hybrid optoelectronic devices that combine the advantages of both optical and electronic technologies, leading to improved performance and functionality in various applications.
- Photonic integrated circuits (PICs): Silicon photonics facilitates the development of photonic integrated circuits, which are analogous to electronic integrated circuits but use light instead of electrons. PICs can incorporate multiple optical functions on a single chip, including light generation, modulation, detection, and routing, enabling complex optical systems to be miniaturized and integrated.
- Applications in quantum computing and sensing: Silicon photonics technology is being applied to quantum computing and sensing applications. It enables the development of quantum photonic devices, such as single-photon sources and detectors, which are crucial components for quantum information processing and quantum sensing systems.
02 Optical interconnects and data transmission
Silicon photonics is utilized in developing high-speed optical interconnects for data centers and telecommunications networks. These interconnects use light for data transmission, offering higher bandwidth and lower power consumption compared to traditional electronic interconnects. The technology enables efficient data transfer between chips, boards, and systems, addressing the growing demand for faster and more energy-efficient communication.Expand Specific Solutions03 Photonic integrated circuits (PICs)
Silicon photonics allows for the creation of complex photonic integrated circuits that combine multiple optical functions on a single chip. These PICs can include lasers, modulators, multiplexers, and detectors, enabling advanced functionalities such as wavelength division multiplexing and optical signal processing. The integration of these components on silicon platforms offers cost-effective and scalable solutions for various applications.Expand Specific Solutions04 Silicon-based light sources and modulators
Advancements in silicon photonics have led to the development of efficient light sources and modulators integrated on silicon chips. This includes the creation of silicon-based lasers, light-emitting diodes, and high-speed electro-optic modulators. These components are crucial for enabling on-chip optical communication and signal processing, overcoming the limitations of traditional silicon-based electronics.Expand Specific Solutions05 Photonic-electronic integration
Silicon photonics technology enables the seamless integration of photonic and electronic components on a single chip or package. This integration allows for the development of hybrid systems that combine the advantages of both optical and electronic technologies. Such integration facilitates improved performance in areas like optical computing, neuromorphic computing, and high-speed analog-to-digital conversion.Expand Specific Solutions
Key Players in Silicon Photonics and Wearables
Silicon photonics is emerging as a transformative technology in the wearable medical device market, currently in its early growth stage. The market is expanding rapidly, driven by increasing demand for non-invasive health monitoring solutions. While the technology is still maturing, major players like Huawei, Google, and IBM are investing heavily in research and development. Companies such as Rockley Photonics and Lumentum are focusing on specialized applications, while semiconductor giants like TSMC and GlobalFoundries are developing manufacturing capabilities. Universities and research institutions, including the University of Washington and IMEC, are contributing to fundamental advancements. As the technology progresses, we can expect increased integration and miniaturization, leading to more sophisticated and accurate wearable medical devices.
Huawei Technologies Co., Ltd.
Technical Solution: Huawei has been actively developing silicon photonics technology with potential applications in wearable medical devices. Their research focuses on creating high-performance, low-power photonic integrated circuits that can be used in various applications, including healthcare. Huawei's silicon photonics solutions aim to improve data transmission speeds and reduce energy consumption, which are crucial for wearable devices[11]. They have demonstrated silicon photonic transceivers capable of data rates up to 400 Gb/s, which could significantly enhance the capabilities of wearable health monitors[12]. For medical applications, Huawei is exploring the use of silicon photonics in biosensors and spectroscopic devices, potentially enabling non-invasive monitoring of various health parameters[13].
Strengths: Strong focus on high-speed data transmission and energy efficiency, potential for integration with 5G and IoT technologies for remote health monitoring. Weaknesses: May face challenges in some markets due to geopolitical concerns, potential need for additional expertise in specific medical applications.
Google LLC
Technical Solution: Google has been actively involved in silicon photonics research, with potential applications in wearable medical devices. Their work includes developing compact, energy-efficient optical switches and modulators that could be integrated into wearable health monitoring systems[6]. Google's silicon photonics technology focuses on improving data transmission speeds and reducing power consumption, which are crucial for wearable devices. They have demonstrated silicon photonic chips capable of transmitting data at speeds up to 100 Gb/s while consuming minimal power[7]. For wearable medical devices, this technology could enable real-time, high-bandwidth data transmission between the device and cloud-based analytics platforms, enhancing the capabilities of remote health monitoring systems.
Strengths: Strong focus on energy efficiency and high-speed data transmission, potential for integration with cloud-based health analytics platforms. Weaknesses: May lack specific focus on medical applications, potential privacy concerns with data handling.
Breakthrough Silicon Photonics Technologies
Photonic device
PatentWO2021061890A1
Innovation
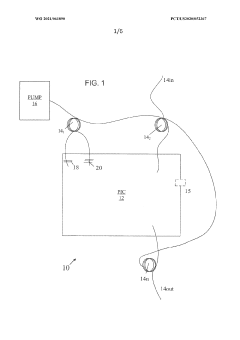
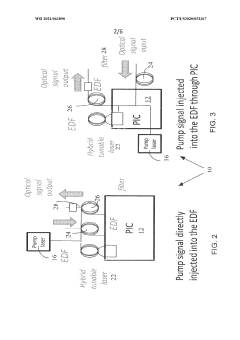
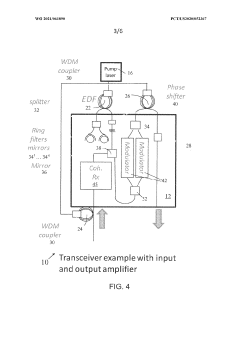
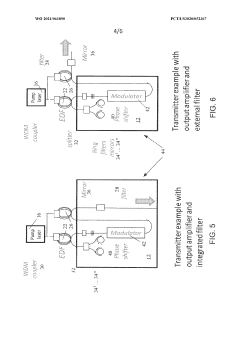
- Integration of a fiber-based gain medium with a photonic integrated circuit (PIC) using a non-hermetic configuration, where the fiber link is free from active and passive photonic elements, allowing for a single external pump to energize multiple fiber gain media, forming a hybrid resonant cavity with low power consumption and high output power.
Photonic devices integrated with thermally conductive layers
PatentActiveUS11934021B2
Innovation
- Incorporating electrically isolated thermally conductive layers above a substrate, with optoelectronic components spaced apart from these layers to facilitate heat dissipation while minimizing interference with optical signals, using materials like metal semiconductor compounds with high thermal conductivity.
Regulatory Framework for Photonic Medical Devices
The regulatory framework for photonic medical devices is a critical aspect of the development and deployment of silicon photonics in wearable medical applications. As these technologies advance, regulatory bodies worldwide are adapting their guidelines to ensure the safety and efficacy of these innovative devices.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating photonic medical devices. The FDA has established specific guidelines for optical diagnostic devices, which include many silicon photonics-based wearables. These guidelines cover aspects such as optical safety, biocompatibility, and performance standards. Manufacturers must demonstrate compliance with these regulations through rigorous testing and documentation before obtaining market approval.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation includes specific provisions for active medical devices, encompassing many photonic wearables. The MDR emphasizes a life-cycle approach to device safety and performance, requiring manufacturers to conduct post-market surveillance and report adverse events.
In Asia, countries like Japan and China have also developed regulatory frameworks for photonic medical devices. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established guidelines for optical medical equipment, while China's National Medical Products Administration (NMPA) has implemented regulations specific to innovative medical technologies, including photonics-based devices.
International standards organizations, such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have developed standards specific to photonic medical devices. These include IEC 60601-2-57 for the safety of light sources used on the skin and ISO 13485 for quality management systems in medical device manufacturing.
As silicon photonics technology advances, regulatory bodies are increasingly focusing on data privacy and cybersecurity aspects of wearable medical devices. This is particularly relevant for devices that transmit patient data wirelessly or connect to mobile applications. Regulations such as the EU's General Data Protection Regulation (GDPR) and the U.S. Health Insurance Portability and Accountability Act (HIPAA) have significant implications for the design and operation of these devices.
The regulatory landscape for photonic medical devices is dynamic, with agencies continuously updating their guidelines to keep pace with technological advancements. Manufacturers and developers in the field of silicon photonics for wearable medical devices must maintain close collaboration with regulatory bodies to ensure compliance throughout the product lifecycle. This ongoing dialogue between industry and regulators is crucial for fostering innovation while safeguarding patient safety and public health.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating photonic medical devices. The FDA has established specific guidelines for optical diagnostic devices, which include many silicon photonics-based wearables. These guidelines cover aspects such as optical safety, biocompatibility, and performance standards. Manufacturers must demonstrate compliance with these regulations through rigorous testing and documentation before obtaining market approval.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation includes specific provisions for active medical devices, encompassing many photonic wearables. The MDR emphasizes a life-cycle approach to device safety and performance, requiring manufacturers to conduct post-market surveillance and report adverse events.
In Asia, countries like Japan and China have also developed regulatory frameworks for photonic medical devices. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established guidelines for optical medical equipment, while China's National Medical Products Administration (NMPA) has implemented regulations specific to innovative medical technologies, including photonics-based devices.
International standards organizations, such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have developed standards specific to photonic medical devices. These include IEC 60601-2-57 for the safety of light sources used on the skin and ISO 13485 for quality management systems in medical device manufacturing.
As silicon photonics technology advances, regulatory bodies are increasingly focusing on data privacy and cybersecurity aspects of wearable medical devices. This is particularly relevant for devices that transmit patient data wirelessly or connect to mobile applications. Regulations such as the EU's General Data Protection Regulation (GDPR) and the U.S. Health Insurance Portability and Accountability Act (HIPAA) have significant implications for the design and operation of these devices.
The regulatory landscape for photonic medical devices is dynamic, with agencies continuously updating their guidelines to keep pace with technological advancements. Manufacturers and developers in the field of silicon photonics for wearable medical devices must maintain close collaboration with regulatory bodies to ensure compliance throughout the product lifecycle. This ongoing dialogue between industry and regulators is crucial for fostering innovation while safeguarding patient safety and public health.
Biocompatibility and Safety Considerations
The integration of silicon photonics in wearable medical devices necessitates careful consideration of biocompatibility and safety aspects. Silicon, while generally considered biocompatible, may still pose potential risks when used in close contact with human tissues for extended periods. The biocompatibility of silicon-based devices depends on various factors, including surface properties, device design, and the specific application.
One crucial aspect of biocompatibility is the potential for silicon particles to elicit inflammatory responses or cause tissue irritation. To mitigate these risks, researchers are exploring surface modification techniques, such as coating silicon surfaces with biocompatible materials like titanium dioxide or hydroxyapatite. These coatings can enhance the overall biocompatibility of the device and reduce the likelihood of adverse reactions.
Another important consideration is the long-term stability of silicon-based devices in physiological environments. Exposure to bodily fluids and varying pH levels can potentially lead to degradation of silicon components over time. This degradation may not only compromise device performance but also release potentially harmful byproducts. To address this issue, researchers are developing protective encapsulation methods and investigating more resistant silicon alloys.
The miniaturization of silicon photonic components in wearable medical devices also raises concerns about the potential for nanoparticle release. As devices become smaller and more integrated with the body, the risk of nanoparticle shedding increases. These nanoparticles could potentially enter the bloodstream or accumulate in tissues, leading to unforeseen health consequences. Ongoing research is focused on developing robust containment strategies and assessing the long-term effects of silicon nanoparticle exposure.
Electromagnetic compatibility is another critical safety consideration for silicon photonic devices in medical applications. These devices often operate in close proximity to other electronic medical equipment and must not interfere with their function. Careful shielding and design considerations are necessary to ensure that silicon photonic components do not emit electromagnetic radiation that could disrupt other devices or pose risks to patients.
Thermal management is also a key safety concern, particularly for wearable devices that operate in direct contact with the skin. Silicon photonic components can generate heat during operation, which must be effectively dissipated to prevent tissue damage or discomfort. Innovative cooling solutions and thermal management strategies are being developed to address this challenge and ensure safe, long-term use of these devices.
As the field of silicon photonics in wearable medical devices continues to advance, regulatory bodies are adapting their guidelines to address these emerging biocompatibility and safety considerations. Manufacturers and researchers must work closely with regulatory agencies to establish comprehensive testing protocols and safety standards specific to silicon photonic medical devices. This collaboration will be crucial in ensuring the safe and effective implementation of this technology in healthcare applications.
One crucial aspect of biocompatibility is the potential for silicon particles to elicit inflammatory responses or cause tissue irritation. To mitigate these risks, researchers are exploring surface modification techniques, such as coating silicon surfaces with biocompatible materials like titanium dioxide or hydroxyapatite. These coatings can enhance the overall biocompatibility of the device and reduce the likelihood of adverse reactions.
Another important consideration is the long-term stability of silicon-based devices in physiological environments. Exposure to bodily fluids and varying pH levels can potentially lead to degradation of silicon components over time. This degradation may not only compromise device performance but also release potentially harmful byproducts. To address this issue, researchers are developing protective encapsulation methods and investigating more resistant silicon alloys.
The miniaturization of silicon photonic components in wearable medical devices also raises concerns about the potential for nanoparticle release. As devices become smaller and more integrated with the body, the risk of nanoparticle shedding increases. These nanoparticles could potentially enter the bloodstream or accumulate in tissues, leading to unforeseen health consequences. Ongoing research is focused on developing robust containment strategies and assessing the long-term effects of silicon nanoparticle exposure.
Electromagnetic compatibility is another critical safety consideration for silicon photonic devices in medical applications. These devices often operate in close proximity to other electronic medical equipment and must not interfere with their function. Careful shielding and design considerations are necessary to ensure that silicon photonic components do not emit electromagnetic radiation that could disrupt other devices or pose risks to patients.
Thermal management is also a key safety concern, particularly for wearable devices that operate in direct contact with the skin. Silicon photonic components can generate heat during operation, which must be effectively dissipated to prevent tissue damage or discomfort. Innovative cooling solutions and thermal management strategies are being developed to address this challenge and ensure safe, long-term use of these devices.
As the field of silicon photonics in wearable medical devices continues to advance, regulatory bodies are adapting their guidelines to address these emerging biocompatibility and safety considerations. Manufacturers and researchers must work closely with regulatory agencies to establish comprehensive testing protocols and safety standards specific to silicon photonic medical devices. This collaboration will be crucial in ensuring the safe and effective implementation of this technology in healthcare applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!