The Role of Microinjection Molding in Drug Delivery Systems
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microinjection Molding Evolution and Objectives
Microinjection molding technology has evolved significantly since its inception in the late 1980s, initially developed as a specialized adaptation of conventional injection molding to address the growing demand for miniaturized components. The technology emerged primarily from the electronics and semiconductor industries, where the need for increasingly smaller components drove innovation in manufacturing processes. By the early 1990s, microinjection molding began to attract attention from the medical device sector, particularly for applications requiring high-precision, small-scale components.
The evolution of microinjection molding has been characterized by continuous improvements in machine precision, material science, and process control. Early systems faced significant limitations in terms of shot weight control, material flow behavior at the micro scale, and tooling precision. The 2000s marked a turning point with the introduction of specialized microinjection molding machines featuring improved screw designs, precise dosing systems, and enhanced control algorithms that enabled more reliable production of micro components.
Material development has played a crucial role in this evolution, with the introduction of biocompatible polymers specifically formulated for microinjection molding. These materials exhibit optimized flow properties at the micro scale while maintaining the mechanical, chemical, and biological properties required for drug delivery applications. The advancement from simple thermoplastics to high-performance polymers, including biodegradable options, has significantly expanded the potential applications in drug delivery systems.
In the context of drug delivery systems, microinjection molding aims to achieve several critical objectives. Primarily, it seeks to enable the mass production of complex, miniaturized drug delivery components with unprecedented precision and reproducibility. This includes microneedles, microfluidic channels, reservoirs, and controlled-release mechanisms that operate at the microscale. The technology aims to achieve feature sizes in the micrometer range while maintaining tight tolerances, often below 10 micrometers.
Another key objective is to facilitate the integration of multiple functionalities within a single component, reducing assembly steps and enhancing reliability. This includes the development of multi-material microinjection molding techniques that can combine different polymers with varying properties in a single part, enabling sophisticated drug release mechanisms.
Cost-effectiveness represents another crucial objective, as microinjection molding strives to make advanced drug delivery technologies economically viable for widespread adoption. The technology aims to reduce material waste, increase production efficiency, and lower the overall manufacturing costs of complex drug delivery systems, making innovative treatments more accessible to patients globally.
Looking forward, microinjection molding technology continues to evolve toward enabling increasingly sophisticated drug delivery systems with enhanced capabilities for targeted delivery, controlled release profiles, and patient-specific customization. The integration with other technologies, such as 3D printing and advanced surface treatments, represents the frontier of this technological evolution.
The evolution of microinjection molding has been characterized by continuous improvements in machine precision, material science, and process control. Early systems faced significant limitations in terms of shot weight control, material flow behavior at the micro scale, and tooling precision. The 2000s marked a turning point with the introduction of specialized microinjection molding machines featuring improved screw designs, precise dosing systems, and enhanced control algorithms that enabled more reliable production of micro components.
Material development has played a crucial role in this evolution, with the introduction of biocompatible polymers specifically formulated for microinjection molding. These materials exhibit optimized flow properties at the micro scale while maintaining the mechanical, chemical, and biological properties required for drug delivery applications. The advancement from simple thermoplastics to high-performance polymers, including biodegradable options, has significantly expanded the potential applications in drug delivery systems.
In the context of drug delivery systems, microinjection molding aims to achieve several critical objectives. Primarily, it seeks to enable the mass production of complex, miniaturized drug delivery components with unprecedented precision and reproducibility. This includes microneedles, microfluidic channels, reservoirs, and controlled-release mechanisms that operate at the microscale. The technology aims to achieve feature sizes in the micrometer range while maintaining tight tolerances, often below 10 micrometers.
Another key objective is to facilitate the integration of multiple functionalities within a single component, reducing assembly steps and enhancing reliability. This includes the development of multi-material microinjection molding techniques that can combine different polymers with varying properties in a single part, enabling sophisticated drug release mechanisms.
Cost-effectiveness represents another crucial objective, as microinjection molding strives to make advanced drug delivery technologies economically viable for widespread adoption. The technology aims to reduce material waste, increase production efficiency, and lower the overall manufacturing costs of complex drug delivery systems, making innovative treatments more accessible to patients globally.
Looking forward, microinjection molding technology continues to evolve toward enabling increasingly sophisticated drug delivery systems with enhanced capabilities for targeted delivery, controlled release profiles, and patient-specific customization. The integration with other technologies, such as 3D printing and advanced surface treatments, represents the frontier of this technological evolution.
Market Analysis for Precision Drug Delivery Systems
The global market for precision drug delivery systems has experienced significant growth in recent years, driven by increasing prevalence of chronic diseases, technological advancements, and growing demand for minimally invasive drug administration methods. The market was valued at approximately 35 billion USD in 2022 and is projected to reach 71 billion USD by 2030, representing a compound annual growth rate (CAGR) of 9.3% during the forecast period.
Microinjection molding technology has emerged as a critical enabler in this market, particularly for manufacturing complex micro-components used in advanced drug delivery systems. The precision components segment, which includes microinjection-molded parts, currently accounts for about 18% of the overall drug delivery systems market and is expected to grow at a higher rate than the market average.
North America dominates the precision drug delivery systems market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (21%). However, the Asia-Pacific region is anticipated to witness the fastest growth due to increasing healthcare expenditure, growing medical tourism, and expanding pharmaceutical manufacturing capabilities in countries like China, India, and South Korea.
By application type, the market can be segmented into implantable systems, wearable devices, inhalation devices, transdermal systems, and injectable systems. Injectable systems, where microinjection molding plays a crucial role, represent the largest segment with 34% market share, followed by implantable systems at 27%.
The demand for personalized medicine is creating significant opportunities for precision drug delivery systems. According to recent market surveys, 78% of healthcare providers believe that personalized drug delivery will become standard practice within the next decade, driving further innovation in microinjection molding technologies.
Key market drivers include the rising geriatric population, increasing incidence of diabetes and cancer, growing preference for self-administration devices, and technological advancements in polymer materials compatible with biological drugs. The shift toward biologics and biosimilars has particularly accelerated the need for sophisticated delivery mechanisms that can handle sensitive biomolecules.
Market challenges include stringent regulatory requirements, high development costs, and technical complexities in manufacturing microscale components with the required precision. The average development timeline for a new precision drug delivery system is approximately 3-5 years, with regulatory approval processes accounting for a significant portion of this timeframe.
Customer preferences are increasingly favoring smart drug delivery systems with connectivity features, dose monitoring capabilities, and improved user interfaces. This trend is expected to drive further integration of microinjection-molded components with electronic elements, creating new market opportunities for manufacturers with cross-disciplinary expertise.
Microinjection molding technology has emerged as a critical enabler in this market, particularly for manufacturing complex micro-components used in advanced drug delivery systems. The precision components segment, which includes microinjection-molded parts, currently accounts for about 18% of the overall drug delivery systems market and is expected to grow at a higher rate than the market average.
North America dominates the precision drug delivery systems market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (21%). However, the Asia-Pacific region is anticipated to witness the fastest growth due to increasing healthcare expenditure, growing medical tourism, and expanding pharmaceutical manufacturing capabilities in countries like China, India, and South Korea.
By application type, the market can be segmented into implantable systems, wearable devices, inhalation devices, transdermal systems, and injectable systems. Injectable systems, where microinjection molding plays a crucial role, represent the largest segment with 34% market share, followed by implantable systems at 27%.
The demand for personalized medicine is creating significant opportunities for precision drug delivery systems. According to recent market surveys, 78% of healthcare providers believe that personalized drug delivery will become standard practice within the next decade, driving further innovation in microinjection molding technologies.
Key market drivers include the rising geriatric population, increasing incidence of diabetes and cancer, growing preference for self-administration devices, and technological advancements in polymer materials compatible with biological drugs. The shift toward biologics and biosimilars has particularly accelerated the need for sophisticated delivery mechanisms that can handle sensitive biomolecules.
Market challenges include stringent regulatory requirements, high development costs, and technical complexities in manufacturing microscale components with the required precision. The average development timeline for a new precision drug delivery system is approximately 3-5 years, with regulatory approval processes accounting for a significant portion of this timeframe.
Customer preferences are increasingly favoring smart drug delivery systems with connectivity features, dose monitoring capabilities, and improved user interfaces. This trend is expected to drive further integration of microinjection-molded components with electronic elements, creating new market opportunities for manufacturers with cross-disciplinary expertise.
Technical Challenges in Microinjection Molding for Pharmaceuticals
Microinjection molding for pharmaceutical applications faces several significant technical challenges that must be overcome to fully realize its potential in drug delivery systems. The primary challenge lies in achieving precise dimensional control at the microscale. When manufacturing components with features in the micrometer range, even minor variations can significantly impact drug delivery performance. Tolerances that would be acceptable in conventional molding become critical barriers in microinjection molding, requiring advanced process control systems and metrology techniques.
Material selection presents another substantial hurdle. Polymers used in pharmaceutical applications must meet stringent biocompatibility and regulatory requirements while simultaneously possessing the flow characteristics necessary for filling microscale cavities. The rheological behavior of polymers changes dramatically at the microscale, with surface tension and cooling rate effects becoming dominant factors. This necessitates specialized material formulations and processing parameters that differ significantly from conventional molding approaches.
Tool design and fabrication represent perhaps the most technically demanding aspect of microinjection molding. Creating mold cavities with microscale features requires advanced manufacturing techniques such as micro-electrical discharge machining, laser ablation, or LIGA processes. These tools must maintain exceptional surface finish quality and dimensional stability under repeated high-pressure injection cycles. Tool wear becomes particularly problematic at this scale, as even nanometer-level degradation can impact part quality.
Process control challenges are equally significant. The injection phase occurs in milliseconds, requiring ultra-precise control systems with response times orders of magnitude faster than conventional molding equipment. Sensors must detect and respond to variations in pressure, temperature, and flow rate with unprecedented sensitivity. The process window—the range of parameters yielding acceptable parts—becomes extremely narrow, making consistent production difficult to maintain.
Post-molding operations introduce additional complexities. Ejection of microparts without damage requires specialized techniques, as conventional ejector pins may be larger than the parts themselves. Quality inspection becomes extraordinarily challenging, often requiring scanning electron microscopy or other advanced imaging techniques to verify critical dimensions and surface characteristics.
Integration challenges emerge when incorporating active pharmaceutical ingredients (APIs) into the molding process. Temperature-sensitive drugs may degrade during processing, while maintaining homogeneous distribution of APIs throughout the polymer matrix requires specialized mixing and processing approaches. The interaction between polymer and drug compounds must be thoroughly characterized to ensure stability throughout the product lifecycle.
Scaling production from laboratory to commercial volumes introduces yet another layer of technical difficulty. Maintaining process consistency across multiple cavities and machines requires sophisticated statistical process control methods and may necessitate custom-designed equipment that differs substantially from conventional molding systems.
Material selection presents another substantial hurdle. Polymers used in pharmaceutical applications must meet stringent biocompatibility and regulatory requirements while simultaneously possessing the flow characteristics necessary for filling microscale cavities. The rheological behavior of polymers changes dramatically at the microscale, with surface tension and cooling rate effects becoming dominant factors. This necessitates specialized material formulations and processing parameters that differ significantly from conventional molding approaches.
Tool design and fabrication represent perhaps the most technically demanding aspect of microinjection molding. Creating mold cavities with microscale features requires advanced manufacturing techniques such as micro-electrical discharge machining, laser ablation, or LIGA processes. These tools must maintain exceptional surface finish quality and dimensional stability under repeated high-pressure injection cycles. Tool wear becomes particularly problematic at this scale, as even nanometer-level degradation can impact part quality.
Process control challenges are equally significant. The injection phase occurs in milliseconds, requiring ultra-precise control systems with response times orders of magnitude faster than conventional molding equipment. Sensors must detect and respond to variations in pressure, temperature, and flow rate with unprecedented sensitivity. The process window—the range of parameters yielding acceptable parts—becomes extremely narrow, making consistent production difficult to maintain.
Post-molding operations introduce additional complexities. Ejection of microparts without damage requires specialized techniques, as conventional ejector pins may be larger than the parts themselves. Quality inspection becomes extraordinarily challenging, often requiring scanning electron microscopy or other advanced imaging techniques to verify critical dimensions and surface characteristics.
Integration challenges emerge when incorporating active pharmaceutical ingredients (APIs) into the molding process. Temperature-sensitive drugs may degrade during processing, while maintaining homogeneous distribution of APIs throughout the polymer matrix requires specialized mixing and processing approaches. The interaction between polymer and drug compounds must be thoroughly characterized to ensure stability throughout the product lifecycle.
Scaling production from laboratory to commercial volumes introduces yet another layer of technical difficulty. Maintaining process consistency across multiple cavities and machines requires sophisticated statistical process control methods and may necessitate custom-designed equipment that differs substantially from conventional molding systems.
Current Microinjection Molding Solutions for Drug Delivery
01 Microinjection molding equipment and machinery
Specialized equipment and machinery designed specifically for microinjection molding processes. These include miniaturized injection units, precision molds, and automated systems that enable the production of micro-scale components with high accuracy. The equipment often features advanced control systems for precise parameter management and specialized ejection mechanisms suitable for delicate micro parts.- Equipment and tooling for microinjection molding: Specialized equipment and tooling are essential for microinjection molding processes. This includes micro-molds with precise cavity designs, advanced injection units capable of delivering small shot sizes with high accuracy, and specialized clamping systems. The equipment often features enhanced control systems for temperature, pressure, and injection speed to ensure the production of high-quality micro components with tight tolerances.
- Materials for microinjection molding: Various materials are used in microinjection molding, including specialized polymers with high flow properties, biocompatible materials for medical applications, and engineered resins with enhanced mechanical properties. Material selection is critical as it affects flow behavior in micro-cavities, part quality, and final product performance. Advanced polymer blends and composites are developed specifically to meet the challenges of filling micro-scale features while maintaining dimensional stability.
- Process parameters and optimization: Optimizing process parameters is crucial for successful microinjection molding. This includes precise control of injection speed, pressure profiles, melt temperature, mold temperature, and cooling rates. Advanced simulation tools help predict material flow behavior in micro-cavities and optimize process windows. Statistical methods like Design of Experiments (DOE) are employed to identify optimal processing conditions that minimize defects while maximizing part quality and production efficiency.
- Biomedical applications of microinjection molding: Microinjection molding is widely used in biomedical applications for producing components like microfluidic devices, lab-on-a-chip systems, drug delivery devices, and diagnostic tools. The process enables the production of complex micro-structures with biocompatible materials that meet stringent medical requirements. Special considerations include clean room manufacturing environments, validation protocols, and quality control measures to ensure product safety and efficacy for medical use.
- Advanced technologies and innovations: Recent innovations in microinjection molding include multi-material processing, in-mold assembly techniques, and integration with other micro-manufacturing methods. Technologies like variothermal process control, vacuum-assisted molding, and ultrasonic-assisted injection enhance the capability to produce increasingly complex micro-parts. Industry 4.0 concepts are being implemented with real-time monitoring systems, artificial intelligence for process control, and automated quality inspection to improve consistency and reduce cycle times in microinjection molding operations.
02 Materials for microinjection molding
Various materials specifically formulated or selected for microinjection molding applications. These include specialized polymers, biocompatible materials, and composite materials that exhibit favorable flow characteristics at the micro scale. The materials are designed to maintain structural integrity while filling microscopic cavities and features, with considerations for shrinkage, surface finish, and mechanical properties in the final micro components.Expand Specific Solutions03 Biomedical applications of microinjection molding
Implementation of microinjection molding techniques in the biomedical field for producing medical devices, diagnostic tools, and drug delivery systems. This includes the fabrication of microfluidic devices, microneedles, implantable components, and lab-on-a-chip systems. The process enables the production of complex geometries at microscale with biocompatible materials suitable for medical applications.Expand Specific Solutions04 Process optimization for microinjection molding
Methods and techniques for optimizing the microinjection molding process to achieve higher precision, better quality, and increased production efficiency. This includes parameter optimization (temperature, pressure, injection speed), cycle time reduction, defect minimization, and process monitoring strategies. Advanced simulation tools and experimental design approaches are used to determine optimal processing conditions for specific micro components.Expand Specific Solutions05 Novel microinjection molding technologies
Emerging and innovative technologies in the field of microinjection molding that extend beyond conventional approaches. These include hybrid processes combining microinjection with other manufacturing techniques, multi-material microinjection molding, in-mold assembly, and advanced surface treatment methods. These novel technologies enable the production of increasingly complex micro components with enhanced functionality and performance characteristics.Expand Specific Solutions
Industry Leaders in Microinjection Molding Technologies
Microinjection molding in drug delivery systems is evolving rapidly in a market transitioning from early growth to maturity. The global market is expanding significantly, driven by demand for precise, minimally invasive drug delivery solutions. Technologically, the field shows varying maturity levels across players. Leading innovators include Becton Dickinson and Novartis, who have established commercial applications, while academic institutions like MIT, Johns Hopkins, and Huazhong University contribute fundamental research. Specialized firms such as Juvic Co. and Tecmed are advancing microneedle technologies, while 3M and LTS LOHMANN focus on transdermal delivery systems. The competitive landscape features both pharmaceutical giants and agile startups collaborating with research institutions to overcome manufacturing challenges and regulatory hurdles.
Massachusetts Institute of Technology
Technical Solution: MIT has developed groundbreaking microinjection molding technologies for next-generation drug delivery systems through their Langer Lab and other research groups. Their approach utilizes advanced MEMS-based microinjection molding techniques to create complex three-dimensional microstructures with feature sizes down to 2 microns. MIT researchers have pioneered the use of stimuli-responsive polymers in microinjection molding, enabling the creation of drug delivery systems that respond to specific environmental triggers such as pH, temperature, or enzymatic activity. Their technology incorporates multi-layer microinjection molding processes that allow for the creation of compartmentalized delivery systems with programmable release profiles for multiple therapeutic agents. MIT has developed specialized surface modification techniques that can be applied post-molding to enhance biocompatibility and control drug diffusion kinetics. Their research has demonstrated successful encapsulation of biologics including proteins and nucleic acids within micro-molded structures while maintaining therapeutic activity, achieving encapsulation efficiencies exceeding 85% for sensitive biomolecules[6][9].
Strengths: Cutting-edge innovation in materials and manufacturing processes; interdisciplinary approach combining engineering, materials science, and pharmacology; strong academic-industry partnerships facilitating technology transfer. Weaknesses: Technologies often at early development stages requiring significant translation for commercial application; higher complexity in manufacturing processes; intellectual property considerations across multiple stakeholders.
Novartis AG
Technical Solution: Novartis AG has pioneered microinjection molding technology for advanced drug delivery systems through their proprietary NanoMold platform. This technology enables the production of microscale components with feature sizes as small as 5 microns and tolerances of ±2 microns, facilitating the creation of sophisticated controlled-release mechanisms. Their approach combines specialized polymer formulations with multi-cavity microinjection molding processes to achieve high-throughput manufacturing of biodegradable drug carriers. Novartis has developed specialized surface treatment techniques that modify the hydrophilicity/hydrophobicity of micro-molded components to optimize drug loading capacity and release kinetics. Their systems incorporate intelligent design features such as micro-reservoirs with programmable release triggers responding to specific physiological conditions. The company has successfully commercialized several products utilizing this technology, including implantable devices for sustained delivery of ophthalmic medications and targeted oncology therapeutics[2][5].
Strengths: Extensive pharmaceutical formulation expertise integrated with device engineering; strong intellectual property portfolio covering both materials and manufacturing processes; established clinical validation pathways. Weaknesses: High initial capital investment requirements; challenges in scaling production while maintaining tight tolerances; limited application to certain drug classes due to material compatibility constraints.
Key Patents and Innovations in Microinjection Molding
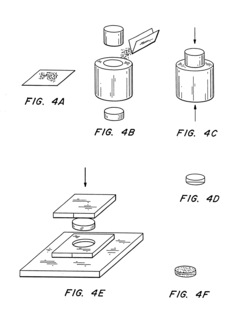
Manufacturing microneedle arrays
PatentInactiveEP1968777A2
Innovation
- A method involving a carrier web with a mold apparatus that injects polymeric material to form microneedle arrays, allowing for handling and processing without direct contact, using air or fluid floatation to move the web and maintain the arrays' integrity, and integrating them into a continuous film backing for further processing and packaging.
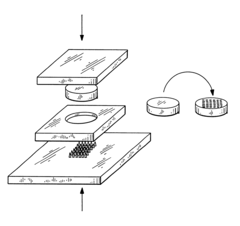
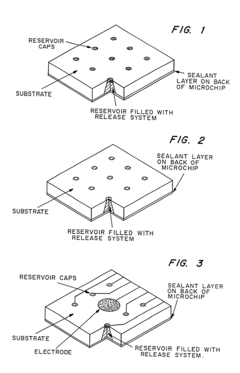
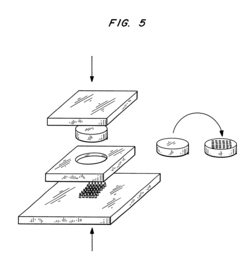
Microchip devices for delivery of molecules and methods of fabrication thereof
PatentInactiveUS6808522B2
Innovation
- Development of miniaturized microchip devices with controlled time and rate release systems using compression molding, injection molding, and microfabrication techniques, allowing for active or passive release of drugs through various materials and stimuli-responsive mechanisms.
Regulatory Compliance for Medical Device Manufacturing
Regulatory compliance represents a critical dimension in the development and commercialization of microinjection molded drug delivery systems. These miniaturized devices must adhere to stringent regulatory frameworks established by authorities such as the FDA in the United States, the EMA in Europe, and similar bodies worldwide. The complexity of compliance increases when considering the dual nature of these products as both medical devices and pharmaceutical delivery mechanisms.
For microinjection molded components in drug delivery systems, manufacturers must navigate multiple regulatory pathways. In the US, these products typically fall under combination product regulations (21 CFR Part 3), requiring compliance with both device regulations (21 CFR Part 820) and pharmaceutical Good Manufacturing Practices (GMPs). The classification of the final product significantly impacts the regulatory burden, with higher-risk devices facing more rigorous scrutiny.
Material selection for microinjection molding presents particular regulatory challenges. All polymers and additives must meet biocompatibility requirements according to ISO 10993 standards, with additional considerations for leachables and extractables when in contact with pharmaceutical compounds. Documentation of material traceability becomes essential, requiring robust supplier qualification processes and material testing protocols.
Manufacturing process validation represents another critical compliance area. The miniaturized nature of microinjection molded components demands exceptional precision and consistency, necessitating comprehensive process validation protocols. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) documentation, along with Statistical Process Control (SPC) methodologies to demonstrate consistent quality production.
Quality management systems specifically tailored to microinjection molding operations must incorporate risk management principles aligned with ISO 14971. This includes Failure Mode and Effects Analysis (FMEA) for both design and process aspects, with particular attention to the unique challenges of micro-scale manufacturing tolerances and their potential impact on drug delivery performance.
International regulatory harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF) initiatives, are gradually streamlining compliance requirements across markets. However, manufacturers must still navigate region-specific requirements, particularly for novel drug delivery technologies utilizing advanced microinjection molding techniques.
Post-market surveillance requirements add another layer of compliance complexity. Manufacturers must implement systems for complaint handling, adverse event reporting, and continuous product monitoring. The miniaturized nature of these components may present unique challenges in failure analysis and root cause determination when field issues arise.
For microinjection molded components in drug delivery systems, manufacturers must navigate multiple regulatory pathways. In the US, these products typically fall under combination product regulations (21 CFR Part 3), requiring compliance with both device regulations (21 CFR Part 820) and pharmaceutical Good Manufacturing Practices (GMPs). The classification of the final product significantly impacts the regulatory burden, with higher-risk devices facing more rigorous scrutiny.
Material selection for microinjection molding presents particular regulatory challenges. All polymers and additives must meet biocompatibility requirements according to ISO 10993 standards, with additional considerations for leachables and extractables when in contact with pharmaceutical compounds. Documentation of material traceability becomes essential, requiring robust supplier qualification processes and material testing protocols.
Manufacturing process validation represents another critical compliance area. The miniaturized nature of microinjection molded components demands exceptional precision and consistency, necessitating comprehensive process validation protocols. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) documentation, along with Statistical Process Control (SPC) methodologies to demonstrate consistent quality production.
Quality management systems specifically tailored to microinjection molding operations must incorporate risk management principles aligned with ISO 14971. This includes Failure Mode and Effects Analysis (FMEA) for both design and process aspects, with particular attention to the unique challenges of micro-scale manufacturing tolerances and their potential impact on drug delivery performance.
International regulatory harmonization efforts, including the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF) initiatives, are gradually streamlining compliance requirements across markets. However, manufacturers must still navigate region-specific requirements, particularly for novel drug delivery technologies utilizing advanced microinjection molding techniques.
Post-market surveillance requirements add another layer of compliance complexity. Manufacturers must implement systems for complaint handling, adverse event reporting, and continuous product monitoring. The miniaturized nature of these components may present unique challenges in failure analysis and root cause determination when field issues arise.
Sustainability Aspects of Microinjection Molding Processes
Sustainability has become a critical consideration in modern manufacturing processes, and microinjection molding for drug delivery systems is no exception. The miniaturization of components in this field offers inherent environmental advantages through significant material reduction compared to conventional manufacturing methods. Typical microinjection molded parts for drug delivery applications require only milligrams of material, representing up to 90% reduction in polymer consumption compared to traditional injection molding processes.
Energy efficiency presents another sustainability dimension worth examining. While microinjection molding requires precise temperature control and high injection pressures, the overall energy footprint is considerably smaller than conventional molding due to reduced material processing volumes and shorter cycle times. Advanced microinjection systems have implemented energy recovery mechanisms and optimized heating/cooling cycles, further reducing energy consumption by 15-30% compared to earlier generation equipment.
Material selection plays a pivotal role in sustainability considerations. Biodegradable polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers are increasingly being utilized in microinjection molding for drug delivery applications. These materials offer end-of-life biodegradability while maintaining the necessary mechanical properties and biocompatibility required for pharmaceutical applications. Recent innovations have also focused on developing bio-based alternatives to petroleum-derived polymers, reducing carbon footprint without compromising performance.
Waste reduction strategies have evolved significantly in microinjection molding processes. Advanced runner systems and gate designs minimize material waste during production, while closed-loop recycling systems enable the reprocessing of sprues and runners. Some manufacturers have reported achieving near-zero waste production through these integrated approaches, particularly significant given the high value of medical-grade polymers used in drug delivery systems.
Water consumption, though less discussed, represents another sustainability aspect of microinjection molding. Modern cooling systems have implemented closed-loop water circulation, reducing freshwater requirements by up to 80% compared to conventional open-loop systems. Additionally, advanced filtration and purification systems allow for water reuse across multiple production cycles, further minimizing environmental impact.
The lifecycle assessment of microinjection molded drug delivery components reveals additional sustainability benefits. The reduced weight and volume of these components translate to lower transportation-related emissions throughout the supply chain. Furthermore, the precision of microinjection molding enables the production of more efficient drug delivery systems that can reduce medication waste and improve therapeutic outcomes, representing sustainability benefits that extend beyond manufacturing into clinical applications.
Energy efficiency presents another sustainability dimension worth examining. While microinjection molding requires precise temperature control and high injection pressures, the overall energy footprint is considerably smaller than conventional molding due to reduced material processing volumes and shorter cycle times. Advanced microinjection systems have implemented energy recovery mechanisms and optimized heating/cooling cycles, further reducing energy consumption by 15-30% compared to earlier generation equipment.
Material selection plays a pivotal role in sustainability considerations. Biodegradable polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers are increasingly being utilized in microinjection molding for drug delivery applications. These materials offer end-of-life biodegradability while maintaining the necessary mechanical properties and biocompatibility required for pharmaceutical applications. Recent innovations have also focused on developing bio-based alternatives to petroleum-derived polymers, reducing carbon footprint without compromising performance.
Waste reduction strategies have evolved significantly in microinjection molding processes. Advanced runner systems and gate designs minimize material waste during production, while closed-loop recycling systems enable the reprocessing of sprues and runners. Some manufacturers have reported achieving near-zero waste production through these integrated approaches, particularly significant given the high value of medical-grade polymers used in drug delivery systems.
Water consumption, though less discussed, represents another sustainability aspect of microinjection molding. Modern cooling systems have implemented closed-loop water circulation, reducing freshwater requirements by up to 80% compared to conventional open-loop systems. Additionally, advanced filtration and purification systems allow for water reuse across multiple production cycles, further minimizing environmental impact.
The lifecycle assessment of microinjection molded drug delivery components reveals additional sustainability benefits. The reduced weight and volume of these components translate to lower transportation-related emissions throughout the supply chain. Furthermore, the precision of microinjection molding enables the production of more efficient drug delivery systems that can reduce medication waste and improve therapeutic outcomes, representing sustainability benefits that extend beyond manufacturing into clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!