Understanding Antifreeze in Complex Multi-Surface Areas
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antifreeze Technology Evolution and Objectives
Antifreeze technology has evolved significantly over the past century, driven by the need to protect various systems and materials from freezing damage in cold environments. The primary objective of antifreeze solutions has been to lower the freezing point of liquids, particularly water, to prevent ice formation and subsequent damage to equipment, structures, and surfaces.
The development of antifreeze technology can be traced back to the early 20th century when the automotive industry began to expand rapidly. The initial focus was on protecting engine cooling systems from freezing during winter months. Ethylene glycol, discovered in 1859, emerged as the primary antifreeze compound due to its effectiveness in lowering the freezing point of water and its relatively low cost of production.
As technology advanced, the applications for antifreeze expanded beyond automotive use. Industries such as aerospace, construction, and energy production began to recognize the importance of freeze protection in their operations. This led to the development of more specialized antifreeze formulations tailored to specific needs and environmental conditions.
In recent decades, environmental concerns have driven research towards more eco-friendly antifreeze solutions. Propylene glycol, a less toxic alternative to ethylene glycol, gained popularity in applications where environmental impact and safety were paramount. Additionally, research into bio-based antifreeze compounds derived from renewable resources has intensified, aiming to reduce reliance on petroleum-based products.
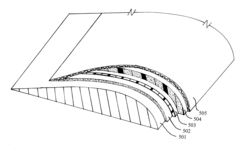
The current technological landscape presents new challenges and opportunities for antifreeze development. The increasing complexity of modern systems and the diverse range of materials used in various industries necessitate more sophisticated antifreeze solutions. Understanding the interaction between antifreeze compounds and different surface materials has become crucial, particularly in complex multi-surface areas where traditional solutions may not provide adequate protection.
The primary objectives of contemporary antifreeze technology research include:
1. Developing more effective and versatile antifreeze formulations capable of protecting a wide range of materials and surfaces simultaneously.
2. Improving the environmental profile of antifreeze solutions by reducing toxicity and enhancing biodegradability.
3. Enhancing the thermal management properties of antifreeze compounds to address both freezing and overheating concerns.
4. Exploring nanotechnology applications to create advanced antifreeze materials with superior performance characteristics.
5. Investigating smart antifreeze systems that can adapt to changing environmental conditions and provide real-time monitoring of freeze protection status.
As we look to the future, the evolution of antifreeze technology will likely focus on addressing these objectives while also considering the broader context of sustainability and energy efficiency. The development of multifunctional antifreeze solutions that can protect complex multi-surface areas while offering additional benefits such as corrosion resistance and thermal conductivity enhancement will be a key area of research and innovation in the coming years.
The development of antifreeze technology can be traced back to the early 20th century when the automotive industry began to expand rapidly. The initial focus was on protecting engine cooling systems from freezing during winter months. Ethylene glycol, discovered in 1859, emerged as the primary antifreeze compound due to its effectiveness in lowering the freezing point of water and its relatively low cost of production.
As technology advanced, the applications for antifreeze expanded beyond automotive use. Industries such as aerospace, construction, and energy production began to recognize the importance of freeze protection in their operations. This led to the development of more specialized antifreeze formulations tailored to specific needs and environmental conditions.
In recent decades, environmental concerns have driven research towards more eco-friendly antifreeze solutions. Propylene glycol, a less toxic alternative to ethylene glycol, gained popularity in applications where environmental impact and safety were paramount. Additionally, research into bio-based antifreeze compounds derived from renewable resources has intensified, aiming to reduce reliance on petroleum-based products.
The current technological landscape presents new challenges and opportunities for antifreeze development. The increasing complexity of modern systems and the diverse range of materials used in various industries necessitate more sophisticated antifreeze solutions. Understanding the interaction between antifreeze compounds and different surface materials has become crucial, particularly in complex multi-surface areas where traditional solutions may not provide adequate protection.
The primary objectives of contemporary antifreeze technology research include:
1. Developing more effective and versatile antifreeze formulations capable of protecting a wide range of materials and surfaces simultaneously.
2. Improving the environmental profile of antifreeze solutions by reducing toxicity and enhancing biodegradability.
3. Enhancing the thermal management properties of antifreeze compounds to address both freezing and overheating concerns.
4. Exploring nanotechnology applications to create advanced antifreeze materials with superior performance characteristics.
5. Investigating smart antifreeze systems that can adapt to changing environmental conditions and provide real-time monitoring of freeze protection status.
As we look to the future, the evolution of antifreeze technology will likely focus on addressing these objectives while also considering the broader context of sustainability and energy efficiency. The development of multifunctional antifreeze solutions that can protect complex multi-surface areas while offering additional benefits such as corrosion resistance and thermal conductivity enhancement will be a key area of research and innovation in the coming years.
Market Analysis for Multi-Surface Antifreeze Solutions
The market for multi-surface antifreeze solutions has experienced significant growth in recent years, driven by increasing demand across various industries. The automotive sector remains a primary consumer, with a growing emphasis on advanced coolant formulations that can protect complex engine systems. Additionally, the aerospace industry has shown a rising interest in specialized antifreeze solutions for aircraft de-icing and hydraulic systems.
Industrial applications have also contributed to market expansion, particularly in regions with extreme climates. The need for effective antifreeze solutions in manufacturing processes, HVAC systems, and industrial machinery has created new opportunities for product development and market penetration. The construction industry has emerged as another key player, utilizing antifreeze additives in concrete mixtures for cold-weather applications.
Market analysis indicates a shift towards environmentally friendly and biodegradable antifreeze formulations. This trend is partly driven by stringent regulations on chemical disposal and increasing consumer awareness of environmental issues. Manufacturers are investing in research and development to create eco-friendly alternatives that maintain high performance standards across multiple surfaces.
The global market for multi-surface antifreeze solutions is geographically diverse, with North America and Europe leading in terms of consumption and technological advancements. However, rapid industrialization and infrastructure development in Asia-Pacific regions, particularly China and India, are expected to drive significant market growth in the coming years.
Competition in the market is intense, with several major players dominating the landscape. These companies are focusing on product innovation, strategic partnerships, and mergers and acquisitions to maintain their market position. Smaller, specialized manufacturers are also carving out niches by offering tailored solutions for specific industries or applications.
Pricing trends in the market have been influenced by fluctuations in raw material costs and increasing research and development expenses. However, the high value-added nature of advanced multi-surface antifreeze solutions has allowed manufacturers to maintain healthy profit margins.
Future market growth is expected to be driven by technological advancements in antifreeze formulations, expanding applications in emerging industries, and the development of smart antifreeze solutions with self-monitoring capabilities. The integration of nanotechnology in antifreeze products is an area of particular interest, promising enhanced performance across a wider range of surfaces and conditions.
Industrial applications have also contributed to market expansion, particularly in regions with extreme climates. The need for effective antifreeze solutions in manufacturing processes, HVAC systems, and industrial machinery has created new opportunities for product development and market penetration. The construction industry has emerged as another key player, utilizing antifreeze additives in concrete mixtures for cold-weather applications.
Market analysis indicates a shift towards environmentally friendly and biodegradable antifreeze formulations. This trend is partly driven by stringent regulations on chemical disposal and increasing consumer awareness of environmental issues. Manufacturers are investing in research and development to create eco-friendly alternatives that maintain high performance standards across multiple surfaces.
The global market for multi-surface antifreeze solutions is geographically diverse, with North America and Europe leading in terms of consumption and technological advancements. However, rapid industrialization and infrastructure development in Asia-Pacific regions, particularly China and India, are expected to drive significant market growth in the coming years.
Competition in the market is intense, with several major players dominating the landscape. These companies are focusing on product innovation, strategic partnerships, and mergers and acquisitions to maintain their market position. Smaller, specialized manufacturers are also carving out niches by offering tailored solutions for specific industries or applications.
Pricing trends in the market have been influenced by fluctuations in raw material costs and increasing research and development expenses. However, the high value-added nature of advanced multi-surface antifreeze solutions has allowed manufacturers to maintain healthy profit margins.
Future market growth is expected to be driven by technological advancements in antifreeze formulations, expanding applications in emerging industries, and the development of smart antifreeze solutions with self-monitoring capabilities. The integration of nanotechnology in antifreeze products is an area of particular interest, promising enhanced performance across a wider range of surfaces and conditions.
Current Challenges in Complex Surface Antifreeze
The development of effective antifreeze solutions for complex multi-surface areas presents several significant challenges. One of the primary obstacles is the diverse nature of surfaces encountered in real-world applications. These surfaces can range from metal and concrete to various polymers and composites, each with unique physical and chemical properties that interact differently with antifreeze compounds.
The heterogeneity of surface materials often leads to inconsistent performance of antifreeze solutions across different areas. What works well on one surface may be ineffective or even detrimental to another. This variability necessitates the development of versatile antifreeze formulations that can maintain efficacy across a wide range of surface types without compromising the integrity of any particular material.
Another critical challenge lies in the dynamic nature of complex multi-surface environments. Temperature fluctuations, humidity changes, and varying exposure to environmental factors can significantly impact the performance of antifreeze solutions. These fluctuations can lead to cyclic freezing and thawing, which may cause mechanical stress and potential damage to surfaces, particularly at interface points between different materials.
The adhesion of antifreeze compounds to diverse surfaces poses another significant hurdle. Ensuring uniform and long-lasting coverage across different surface types is crucial for maintaining consistent antifreeze protection. However, achieving optimal adhesion without compromising the structural or functional properties of the underlying materials remains a complex task.
Environmental concerns and regulatory requirements add another layer of complexity to the development of antifreeze solutions for multi-surface applications. There is an increasing demand for eco-friendly formulations that minimize environmental impact while maintaining high performance. This necessitates the exploration of novel, biodegradable compounds and the reduction of traditional, potentially harmful ingredients.
The scalability of antifreeze solutions for large-scale applications in complex multi-surface areas is also a significant challenge. Developing formulations that can be efficiently and cost-effectively applied over extensive areas, while maintaining consistent performance, requires innovative approaches to both product formulation and application techniques.
Lastly, the long-term durability and stability of antifreeze solutions in complex environments remain areas of ongoing research. Ensuring that these solutions maintain their effectiveness over extended periods, withstanding repeated exposure to various environmental stressors, is crucial for their practical application in real-world scenarios.
The heterogeneity of surface materials often leads to inconsistent performance of antifreeze solutions across different areas. What works well on one surface may be ineffective or even detrimental to another. This variability necessitates the development of versatile antifreeze formulations that can maintain efficacy across a wide range of surface types without compromising the integrity of any particular material.
Another critical challenge lies in the dynamic nature of complex multi-surface environments. Temperature fluctuations, humidity changes, and varying exposure to environmental factors can significantly impact the performance of antifreeze solutions. These fluctuations can lead to cyclic freezing and thawing, which may cause mechanical stress and potential damage to surfaces, particularly at interface points between different materials.
The adhesion of antifreeze compounds to diverse surfaces poses another significant hurdle. Ensuring uniform and long-lasting coverage across different surface types is crucial for maintaining consistent antifreeze protection. However, achieving optimal adhesion without compromising the structural or functional properties of the underlying materials remains a complex task.
Environmental concerns and regulatory requirements add another layer of complexity to the development of antifreeze solutions for multi-surface applications. There is an increasing demand for eco-friendly formulations that minimize environmental impact while maintaining high performance. This necessitates the exploration of novel, biodegradable compounds and the reduction of traditional, potentially harmful ingredients.
The scalability of antifreeze solutions for large-scale applications in complex multi-surface areas is also a significant challenge. Developing formulations that can be efficiently and cost-effectively applied over extensive areas, while maintaining consistent performance, requires innovative approaches to both product formulation and application techniques.
Lastly, the long-term durability and stability of antifreeze solutions in complex environments remain areas of ongoing research. Ensuring that these solutions maintain their effectiveness over extended periods, withstanding repeated exposure to various environmental stressors, is crucial for their practical application in real-world scenarios.
Existing Multi-Surface Antifreeze Solutions
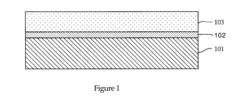
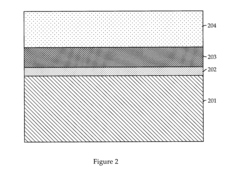
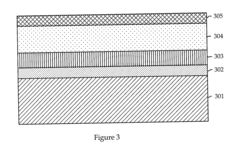
01 Composition of antifreeze solutions
Antifreeze solutions typically consist of a mixture of water and one or more freezing point depressants. Common ingredients include glycols (such as ethylene glycol or propylene glycol), alcohols, and various additives to enhance performance and protect against corrosion. The specific composition affects the freezing point of the solution.- Composition of antifreeze solutions: Antifreeze solutions typically consist of a mixture of water and one or more freezing point depressants. Common ingredients include glycols (such as ethylene glycol or propylene glycol), alcohols, and various additives to enhance performance and protect against corrosion. The specific composition affects the freezing point of the solution.
- Measurement and testing of freezing points: Various methods and devices are used to measure and test the freezing points of antifreeze solutions. These may include specialized instruments, temperature sensors, and analytical techniques to determine the exact temperature at which the solution begins to freeze.
- Additives to lower freezing point: Certain additives can be incorporated into antifreeze formulations to further lower the freezing point. These may include salts, organic compounds, or nanoparticles that enhance the solution's ability to remain liquid at extremely low temperatures.
- Environmental and safety considerations: Development of antifreeze solutions with improved environmental profiles and reduced toxicity is an ongoing area of research. This includes the use of bio-based materials, less toxic alternatives to traditional glycols, and formulations that maintain effectiveness while minimizing environmental impact.
- Application-specific antifreeze formulations: Different applications may require specialized antifreeze formulations with specific freezing point characteristics. These can include automotive coolants, industrial heat transfer fluids, and antifreeze solutions for extreme environments such as aerospace or polar applications.
02 Measurement and testing of freezing points
Various methods and devices are used to measure and test the freezing points of antifreeze solutions. These may include specialized equipment for precise temperature control and monitoring, as well as standardized testing procedures to ensure accuracy and consistency in determining freezing points.Expand Specific Solutions03 Additives for lowering freezing point
Certain additives can be incorporated into antifreeze solutions to further lower the freezing point. These may include salts, organic compounds, or nanoparticles that enhance the solution's ability to remain liquid at lower temperatures. The selection and concentration of these additives are crucial for optimizing the antifreeze performance.Expand Specific Solutions04 Environmental and safety considerations
Development of antifreeze solutions with improved environmental profiles and reduced toxicity is an ongoing area of research. This includes the use of bio-based or biodegradable components, as well as formulations that maintain effective freezing point depression while minimizing potential harm to humans, animals, and ecosystems.Expand Specific Solutions05 Application-specific antifreeze formulations
Different applications may require specialized antifreeze formulations with specific freezing point characteristics. These can include automotive coolants, industrial heat transfer fluids, and antifreeze solutions for renewable energy systems such as solar thermal or geothermal installations. The freezing point requirements vary depending on the intended use and operating conditions.Expand Specific Solutions
Key Players in Advanced Antifreeze Industry
The field of antifreeze technology for complex multi-surface areas is in a mature stage, with a global market size estimated to be in the billions of dollars. Major players like Rolls-Royce, Unilever, and RTX Corp. have established strong positions, leveraging their extensive R&D capabilities. Academic institutions such as MIT, Arizona State University, and Chongqing University contribute significantly to advancing the technology. The market is characterized by ongoing innovation, with companies like Arteco NV and Kaneka Corp. focusing on specialized applications and environmentally friendly solutions. Technological maturity varies across different sectors, with automotive and aerospace industries leading in adoption and refinement of advanced antifreeze solutions.
Rolls-Royce Plc
Technical Solution: Rolls-Royce has developed advanced anti-icing systems for aircraft engines and surfaces, utilizing a combination of electrical heating elements and specialized coatings. Their technology focuses on preventing ice formation in complex multi-surface areas such as engine inlets and wing leading edges. The company employs a multi-layered approach, incorporating electro-thermal ice protection systems (ETIPS) and hydrophobic nano-coatings[1]. These systems work in tandem to create a dynamic ice prevention and removal solution, capable of adapting to various environmental conditions encountered during flight[2].
Strengths: Highly effective in preventing ice formation in critical aircraft components, reducing fuel consumption and enhancing safety. Weaknesses: High power consumption for electrical heating elements, potential for increased aircraft weight.
Unilever Plc
Technical Solution: Unilever has developed innovative antifreeze solutions for complex multi-surface areas in consumer products, particularly in the realm of frozen foods and ice cream. Their approach involves the use of antifreeze proteins (AFPs) derived from arctic fish and plants, which inhibit ice crystal growth and recrystallization[3]. These proteins are incorporated into food formulations to maintain texture and quality during freezing and thawing cycles. Additionally, Unilever has explored the application of these AFPs in non-food products such as cosmetics and personal care items to improve freeze-thaw stability[4]. The company has also invested in research on synthetic antifreeze compounds that mimic the properties of natural AFPs, aiming to enhance efficiency and reduce production costs[5].
Strengths: Natural, food-safe antifreeze solutions with broad applications across multiple product categories. Weaknesses: Potential scalability issues and higher costs compared to traditional antifreeze compounds.
Innovative Antifreeze Formulations for Complex Surfaces
Active Ice-Phobic Freeze Point Reducing Anti-Ice Coating and Method for Providing Anti-Ice Protection to Surfaces
PatentInactiveUS20150299503A1
Innovation
- A superabsorbent polymer coating applied to surfaces, capable of absorbing and retaining at least 50% of an aqueous freeze-point depressant solution, providing extended anti-icing protection by forming a continuous absorbent layer that maintains the solution during adverse weather conditions.
Nanoporous materials, method of manufacture and methods of use
PatentActiveUS20180215927A1
Innovation
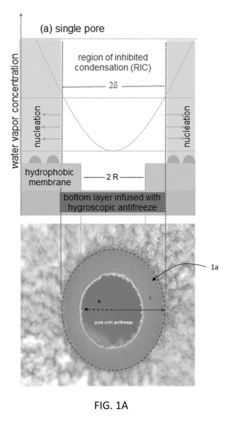
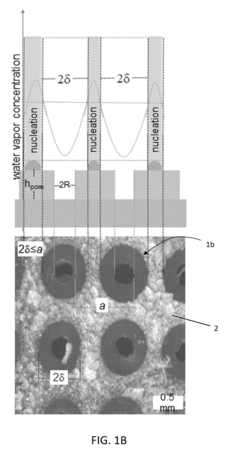
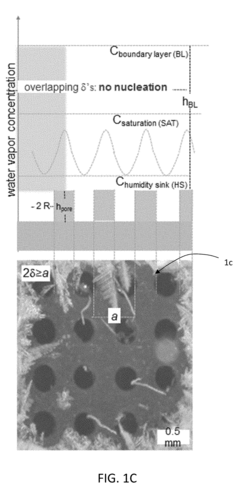
- A bi-layer coating system comprising a hydrophilic or superhydrophilic first layer infused with antifreeze material and a permeable, omniphobic second layer with defined pores, which allows antifreeze migration and creates a localized humidity sink to inhibit frost formation, reducing the need for extensive antifreeze use and enhancing durability.
Environmental Impact of Antifreeze Technologies
The environmental impact of antifreeze technologies is a critical consideration in the development and application of these substances, particularly in complex multi-surface areas. Traditional antifreeze formulations, often based on ethylene glycol or propylene glycol, have raised significant environmental concerns due to their potential toxicity and persistence in ecosystems.
When antifreeze leaks or is improperly disposed of in complex multi-surface areas, it can contaminate soil and water resources. This contamination poses risks to both terrestrial and aquatic life. Ethylene glycol, for instance, is known to be toxic to mammals and can cause severe damage to the central nervous system, heart, and kidneys. In aquatic environments, it can lead to oxygen depletion, harming fish and other organisms.
The biodegradability of antifreeze compounds is a key factor in their environmental impact. While some modern formulations are designed to be more biodegradable, the process can still take time and may produce harmful intermediate compounds. In complex multi-surface areas, the degradation rate can vary significantly depending on factors such as temperature, moisture, and microbial activity.
Another environmental concern is the potential for antifreeze to mobilize heavy metals and other contaminants in soil and groundwater. This is particularly problematic in areas with a history of industrial activity or where multiple surface types intersect, as it can lead to the spread of pollutants beyond their original locations.
The impact on vegetation is also noteworthy. Antifreeze spills can damage or kill plants by altering soil chemistry and disrupting nutrient uptake. In urban environments with diverse surface types, this can affect landscaping, green spaces, and potentially contribute to urban heat island effects.
Efforts to mitigate these environmental impacts have led to the development of more eco-friendly antifreeze formulations. These include propylene glycol-based products, which are less toxic, and plant-based alternatives derived from renewable resources. However, the effectiveness and long-term environmental impacts of these newer formulations in complex multi-surface areas are still subjects of ongoing research.
The proper handling, storage, and disposal of antifreeze are crucial in minimizing environmental risks. This is especially challenging in areas with diverse surface types, where containment and cleanup strategies must be adapted to different materials and drainage patterns. Improved recycling programs and stricter regulations on antifreeze use and disposal are being implemented in many regions to address these challenges.
When antifreeze leaks or is improperly disposed of in complex multi-surface areas, it can contaminate soil and water resources. This contamination poses risks to both terrestrial and aquatic life. Ethylene glycol, for instance, is known to be toxic to mammals and can cause severe damage to the central nervous system, heart, and kidneys. In aquatic environments, it can lead to oxygen depletion, harming fish and other organisms.
The biodegradability of antifreeze compounds is a key factor in their environmental impact. While some modern formulations are designed to be more biodegradable, the process can still take time and may produce harmful intermediate compounds. In complex multi-surface areas, the degradation rate can vary significantly depending on factors such as temperature, moisture, and microbial activity.
Another environmental concern is the potential for antifreeze to mobilize heavy metals and other contaminants in soil and groundwater. This is particularly problematic in areas with a history of industrial activity or where multiple surface types intersect, as it can lead to the spread of pollutants beyond their original locations.
The impact on vegetation is also noteworthy. Antifreeze spills can damage or kill plants by altering soil chemistry and disrupting nutrient uptake. In urban environments with diverse surface types, this can affect landscaping, green spaces, and potentially contribute to urban heat island effects.
Efforts to mitigate these environmental impacts have led to the development of more eco-friendly antifreeze formulations. These include propylene glycol-based products, which are less toxic, and plant-based alternatives derived from renewable resources. However, the effectiveness and long-term environmental impacts of these newer formulations in complex multi-surface areas are still subjects of ongoing research.
The proper handling, storage, and disposal of antifreeze are crucial in minimizing environmental risks. This is especially challenging in areas with diverse surface types, where containment and cleanup strategies must be adapted to different materials and drainage patterns. Improved recycling programs and stricter regulations on antifreeze use and disposal are being implemented in many regions to address these challenges.
Safety Regulations for Antifreeze Applications
The application of antifreeze in complex multi-surface areas necessitates strict adherence to safety regulations to mitigate potential risks and ensure environmental protection. These regulations are designed to address the unique challenges posed by diverse surface materials and varying environmental conditions.
Regulatory bodies, such as the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA), have established comprehensive guidelines for the handling, storage, and disposal of antifreeze substances. These guidelines emphasize the importance of proper containment to prevent spills and leaks, which could lead to contamination of soil and water sources.
In industrial settings, safety regulations mandate the use of appropriate personal protective equipment (PPE) when handling antifreeze. This typically includes chemical-resistant gloves, safety goggles, and protective clothing to minimize the risk of skin contact or inhalation of harmful vapors. Additionally, workplace safety protocols often require the installation of eyewash stations and emergency showers in areas where antifreeze is used or stored.
For automotive applications, regulations focus on the proper disposal of used antifreeze. Many jurisdictions classify spent antifreeze as hazardous waste due to its potential toxicity and environmental impact. As a result, automotive service centers and individuals are required to follow specific disposal procedures, often involving collection by licensed waste management facilities.
In the context of multi-surface areas, safety regulations emphasize the need for compatibility testing between antifreeze formulations and various surface materials. This is particularly crucial in industrial processes where antifreeze may come into contact with different metals, plastics, or composite materials. Manufacturers are required to provide detailed material safety data sheets (MSDS) that outline potential interactions and recommended precautions.
Environmental regulations also play a significant role in antifreeze applications. Many jurisdictions have implemented restrictions on the use of certain antifreeze compounds, particularly those containing ethylene glycol, due to their potential toxicity to wildlife and aquatic ecosystems. As a result, there is a growing trend towards the use of less toxic alternatives, such as propylene glycol-based formulations, especially in areas with sensitive ecological systems.
To ensure compliance with these regulations, companies operating in multi-surface environments are often required to implement comprehensive training programs for employees. These programs cover topics such as proper handling techniques, spill response procedures, and the importance of regular equipment maintenance to prevent leaks and failures.
Regulatory bodies, such as the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA), have established comprehensive guidelines for the handling, storage, and disposal of antifreeze substances. These guidelines emphasize the importance of proper containment to prevent spills and leaks, which could lead to contamination of soil and water sources.
In industrial settings, safety regulations mandate the use of appropriate personal protective equipment (PPE) when handling antifreeze. This typically includes chemical-resistant gloves, safety goggles, and protective clothing to minimize the risk of skin contact or inhalation of harmful vapors. Additionally, workplace safety protocols often require the installation of eyewash stations and emergency showers in areas where antifreeze is used or stored.
For automotive applications, regulations focus on the proper disposal of used antifreeze. Many jurisdictions classify spent antifreeze as hazardous waste due to its potential toxicity and environmental impact. As a result, automotive service centers and individuals are required to follow specific disposal procedures, often involving collection by licensed waste management facilities.
In the context of multi-surface areas, safety regulations emphasize the need for compatibility testing between antifreeze formulations and various surface materials. This is particularly crucial in industrial processes where antifreeze may come into contact with different metals, plastics, or composite materials. Manufacturers are required to provide detailed material safety data sheets (MSDS) that outline potential interactions and recommended precautions.
Environmental regulations also play a significant role in antifreeze applications. Many jurisdictions have implemented restrictions on the use of certain antifreeze compounds, particularly those containing ethylene glycol, due to their potential toxicity to wildlife and aquatic ecosystems. As a result, there is a growing trend towards the use of less toxic alternatives, such as propylene glycol-based formulations, especially in areas with sensitive ecological systems.
To ensure compliance with these regulations, companies operating in multi-surface environments are often required to implement comprehensive training programs for employees. These programs cover topics such as proper handling techniques, spill response procedures, and the importance of regular equipment maintenance to prevent leaks and failures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!