What Innovations Are Emerging in Microinjection Molding for Medical Fields?
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microinjection Molding Evolution and Objectives
Microinjection molding technology has evolved significantly since its inception in the 1980s, transitioning from a niche manufacturing process to a critical technology for producing high-precision medical components. Initially developed as an adaptation of conventional injection molding, this technology has undergone substantial refinement to accommodate the increasing demand for miniaturized medical devices with complex geometries and tight tolerances.
The evolution of microinjection molding can be traced through several distinct phases. The first phase (1980s-1990s) focused on adapting existing injection molding equipment for smaller components, with limited success in achieving true micro-scale features. The second phase (1990s-2000s) saw the development of specialized microinjection molding machines with improved precision control systems and enhanced material handling capabilities.
The third phase (2000s-2010s) marked significant advancements in process control, with the integration of sophisticated sensors and real-time monitoring systems that dramatically improved part consistency and reduced defect rates. Currently, we are in the fourth phase (2010s-present), characterized by the convergence of microinjection molding with other advanced manufacturing technologies, including 3D printing, robotics, and artificial intelligence.
The primary objective of modern microinjection molding in medical applications is to achieve unprecedented levels of precision, consistency, and biocompatibility while reducing production costs and environmental impact. Specific technical goals include developing processes capable of producing features at the sub-micron level, improving surface finish quality to nanometer-scale roughness, and enhancing the moldability of advanced biocompatible and biodegradable polymers.
Another critical objective is the development of multi-material microinjection molding capabilities, allowing for the creation of complex medical devices with integrated functionalities in a single manufacturing step. This includes the incorporation of drug-eluting polymers, antimicrobial materials, and radio-opaque components within the same molded part.
Looking forward, the technology roadmap for microinjection molding in medical applications aims to achieve fully automated, zero-defect manufacturing processes capable of mass-producing highly customized medical components with embedded smart functionalities. This includes the integration of sensors, microfluidic channels, and other functional elements directly into molded parts, enabling the next generation of implantable and diagnostic medical devices.
The convergence of microinjection molding with digital manufacturing paradigms represents the next frontier, with objectives focused on enabling real-time process optimization, predictive quality control, and seamless integration with broader medical device manufacturing ecosystems.
The evolution of microinjection molding can be traced through several distinct phases. The first phase (1980s-1990s) focused on adapting existing injection molding equipment for smaller components, with limited success in achieving true micro-scale features. The second phase (1990s-2000s) saw the development of specialized microinjection molding machines with improved precision control systems and enhanced material handling capabilities.
The third phase (2000s-2010s) marked significant advancements in process control, with the integration of sophisticated sensors and real-time monitoring systems that dramatically improved part consistency and reduced defect rates. Currently, we are in the fourth phase (2010s-present), characterized by the convergence of microinjection molding with other advanced manufacturing technologies, including 3D printing, robotics, and artificial intelligence.
The primary objective of modern microinjection molding in medical applications is to achieve unprecedented levels of precision, consistency, and biocompatibility while reducing production costs and environmental impact. Specific technical goals include developing processes capable of producing features at the sub-micron level, improving surface finish quality to nanometer-scale roughness, and enhancing the moldability of advanced biocompatible and biodegradable polymers.
Another critical objective is the development of multi-material microinjection molding capabilities, allowing for the creation of complex medical devices with integrated functionalities in a single manufacturing step. This includes the incorporation of drug-eluting polymers, antimicrobial materials, and radio-opaque components within the same molded part.
Looking forward, the technology roadmap for microinjection molding in medical applications aims to achieve fully automated, zero-defect manufacturing processes capable of mass-producing highly customized medical components with embedded smart functionalities. This includes the integration of sensors, microfluidic channels, and other functional elements directly into molded parts, enabling the next generation of implantable and diagnostic medical devices.
The convergence of microinjection molding with digital manufacturing paradigms represents the next frontier, with objectives focused on enabling real-time process optimization, predictive quality control, and seamless integration with broader medical device manufacturing ecosystems.
Medical Market Demand Analysis for Micro Components
The global market for micro medical components is experiencing robust growth, driven by increasing demand for minimally invasive surgical procedures, point-of-care diagnostics, and advanced drug delivery systems. Current market valuations indicate the medical microinjection molding sector is expanding at a compound annual growth rate of 11.2%, with particular acceleration in regions with aging populations such as North America, Western Europe, and East Asia.
Miniaturization trends in medical devices represent a primary market driver, with healthcare providers seeking smaller, more precise components that enable less invasive procedures, reduced patient trauma, and shorter recovery times. This trend is particularly evident in cardiovascular, neurological, and ophthalmic applications where sub-millimeter precision is essential for clinical success.
The demand for micro-fluidic devices has surged significantly, with applications in rapid diagnostic testing, lab-on-chip technologies, and personalized medicine platforms. Market research indicates that micro-fluidic components represent the fastest-growing segment within medical microinjection molding, with demand intensified by recent global health challenges requiring rapid, point-of-care testing capabilities.
Drug delivery systems constitute another substantial market segment, with increasing requirements for precise dosing mechanisms, implantable devices, and sustained-release technologies. The shift toward biologics and personalized medicine has created specific demands for micro components capable of delivering complex therapeutic agents with unprecedented precision and reliability.
Material requirements have evolved considerably, with growing demand for biocompatible polymers, high-performance engineering resins, and materials capable of withstanding sterilization processes. Market analysis shows premium pricing potential for components manufactured from advanced materials such as PEEK, liquid crystal polymers, and specialized medical-grade thermoplastics.
Regional market analysis reveals differentiated demand patterns, with North American and European markets prioritizing innovation and performance, while emerging Asian markets emphasize cost-effectiveness alongside quality. Regulatory considerations significantly influence market dynamics, with components requiring FDA, CE marking, or equivalent approvals commanding premium positioning.
The competitive landscape shows increasing consolidation, with medical OEMs preferring suppliers capable of providing integrated solutions rather than individual components. This trend favors microinjection molding specialists with capabilities spanning design optimization, material selection, precision manufacturing, and quality assurance systems aligned with ISO 13485 requirements.
Miniaturization trends in medical devices represent a primary market driver, with healthcare providers seeking smaller, more precise components that enable less invasive procedures, reduced patient trauma, and shorter recovery times. This trend is particularly evident in cardiovascular, neurological, and ophthalmic applications where sub-millimeter precision is essential for clinical success.
The demand for micro-fluidic devices has surged significantly, with applications in rapid diagnostic testing, lab-on-chip technologies, and personalized medicine platforms. Market research indicates that micro-fluidic components represent the fastest-growing segment within medical microinjection molding, with demand intensified by recent global health challenges requiring rapid, point-of-care testing capabilities.
Drug delivery systems constitute another substantial market segment, with increasing requirements for precise dosing mechanisms, implantable devices, and sustained-release technologies. The shift toward biologics and personalized medicine has created specific demands for micro components capable of delivering complex therapeutic agents with unprecedented precision and reliability.
Material requirements have evolved considerably, with growing demand for biocompatible polymers, high-performance engineering resins, and materials capable of withstanding sterilization processes. Market analysis shows premium pricing potential for components manufactured from advanced materials such as PEEK, liquid crystal polymers, and specialized medical-grade thermoplastics.
Regional market analysis reveals differentiated demand patterns, with North American and European markets prioritizing innovation and performance, while emerging Asian markets emphasize cost-effectiveness alongside quality. Regulatory considerations significantly influence market dynamics, with components requiring FDA, CE marking, or equivalent approvals commanding premium positioning.
The competitive landscape shows increasing consolidation, with medical OEMs preferring suppliers capable of providing integrated solutions rather than individual components. This trend favors microinjection molding specialists with capabilities spanning design optimization, material selection, precision manufacturing, and quality assurance systems aligned with ISO 13485 requirements.
Technical Barriers in Medical Microinjection Molding
Despite significant advancements in microinjection molding technology for medical applications, several technical barriers continue to challenge manufacturers and researchers. The miniaturization of components presents fundamental physical limitations, particularly when dealing with part dimensions below 100 microns. At this scale, material flow behavior becomes increasingly unpredictable, with surface tension and cooling rate effects dominating conventional flow dynamics, resulting in incomplete filling of micro-cavities.
Material selection represents another significant challenge. While medical applications demand biocompatible materials that meet stringent regulatory requirements, many suitable polymers exhibit poor flow characteristics at the micro-scale. The limited selection of materials that combine biocompatibility with appropriate rheological properties for microinjection molding restricts innovation and application development.
Tooling precision requirements present extraordinary manufacturing challenges. Micro-molds require extremely tight tolerances, often in the sub-micron range, necessitating specialized manufacturing techniques such as micro-EDM, laser machining, or LIGA processes. These precision requirements dramatically increase tooling costs and lead times, creating significant barriers to entry for smaller manufacturers.
Process control complexity increases exponentially at the micro-scale. Parameters including injection speed, pressure, temperature, and cooling rates must be controlled with unprecedented precision. The processing window—the range of acceptable parameters that yield quality parts—becomes exceedingly narrow, requiring sophisticated monitoring systems and adaptive control algorithms that many manufacturers struggle to implement effectively.
Quality assurance presents unique challenges in microinjection molding for medical applications. Conventional inspection methods prove inadequate for verifying critical dimensions and detecting defects in micro-components. Advanced metrology techniques such as confocal microscopy, white light interferometry, or micro-CT scanning become necessary, significantly increasing quality control costs and complexity.
The integration of additional functionalities into micro-molded medical components, such as drug delivery mechanisms or sensing capabilities, introduces multi-material and overmolding challenges. Achieving reliable bonds between different materials at the micro-scale while maintaining dimensional stability remains technically demanding.
Scalability from prototype to mass production represents a persistent barrier. Processes that work effectively in laboratory settings often fail to translate to high-volume manufacturing environments. The development of robust, repeatable processes that maintain quality across millions of parts requires significant engineering resources and specialized expertise that remains in short supply across the industry.
Material selection represents another significant challenge. While medical applications demand biocompatible materials that meet stringent regulatory requirements, many suitable polymers exhibit poor flow characteristics at the micro-scale. The limited selection of materials that combine biocompatibility with appropriate rheological properties for microinjection molding restricts innovation and application development.
Tooling precision requirements present extraordinary manufacturing challenges. Micro-molds require extremely tight tolerances, often in the sub-micron range, necessitating specialized manufacturing techniques such as micro-EDM, laser machining, or LIGA processes. These precision requirements dramatically increase tooling costs and lead times, creating significant barriers to entry for smaller manufacturers.
Process control complexity increases exponentially at the micro-scale. Parameters including injection speed, pressure, temperature, and cooling rates must be controlled with unprecedented precision. The processing window—the range of acceptable parameters that yield quality parts—becomes exceedingly narrow, requiring sophisticated monitoring systems and adaptive control algorithms that many manufacturers struggle to implement effectively.
Quality assurance presents unique challenges in microinjection molding for medical applications. Conventional inspection methods prove inadequate for verifying critical dimensions and detecting defects in micro-components. Advanced metrology techniques such as confocal microscopy, white light interferometry, or micro-CT scanning become necessary, significantly increasing quality control costs and complexity.
The integration of additional functionalities into micro-molded medical components, such as drug delivery mechanisms or sensing capabilities, introduces multi-material and overmolding challenges. Achieving reliable bonds between different materials at the micro-scale while maintaining dimensional stability remains technically demanding.
Scalability from prototype to mass production represents a persistent barrier. Processes that work effectively in laboratory settings often fail to translate to high-volume manufacturing environments. The development of robust, repeatable processes that maintain quality across millions of parts requires significant engineering resources and specialized expertise that remains in short supply across the industry.
Current Microinjection Molding Solutions for Medical Applications
01 Equipment and apparatus for microinjection molding
Specialized equipment and apparatus are essential for microinjection molding processes. These include micro-molds with precise cavity designs, advanced injection units capable of delivering small material volumes with high accuracy, and specialized clamping systems. The equipment often features enhanced control systems for temperature, pressure, and injection speed to ensure the quality of micro-molded parts. These technological advancements enable the production of complex microstructures with high precision.- Equipment and apparatus for microinjection molding: Specialized equipment and apparatus are essential for microinjection molding processes. These include micro-molds with precise cavity designs, advanced injection units capable of delivering small material volumes with high accuracy, and specialized clamping systems. The equipment often features enhanced temperature control systems, high-precision positioning mechanisms, and specialized ejection systems designed to handle delicate micro-components without damage.
- Materials for microinjection molding: Various materials are used in microinjection molding, including specialized polymers, biocompatible materials, and composite materials with enhanced flow properties. These materials are often modified to improve their flowability at micro-scales, maintain dimensional stability during cooling, and provide specific functional properties in the final micro-components. Material selection is critical for achieving the required mechanical, optical, or electrical properties in micro-molded parts.
- Biomedical applications of microinjection molding: Microinjection molding is widely used in biomedical applications for producing micro-components for medical devices, drug delivery systems, and diagnostic tools. The technology enables the production of precise microfluidic channels, micro-needles, implantable devices, and lab-on-a-chip systems. The process allows for the use of biocompatible materials and can create sterile components with complex geometries at the microscale for various medical applications.
- Process optimization and control in microinjection molding: Optimizing the microinjection molding process involves precise control of processing parameters such as injection speed, pressure, temperature, and cooling rate. Advanced monitoring systems are employed to ensure consistent quality in micro-components. Simulation tools help predict material flow behavior at the micro-scale, while specialized quality control methods are developed to inspect features that may be difficult to measure using conventional techniques. Process optimization is crucial for achieving high precision and repeatability in micro-molded parts.
- Innovative mold designs and fabrication techniques: Advanced mold designs and fabrication techniques are essential for successful microinjection molding. These include micro-machining, laser ablation, LIGA processes, and other precision manufacturing methods to create mold cavities with micro-features. Innovative mold designs incorporate specialized venting systems, optimized gate locations, and advanced cooling channels to address the unique challenges of molding at the micro-scale. These techniques enable the production of increasingly complex and precise micro-components.
02 Materials for microinjection molding
Various materials are used in microinjection molding to achieve specific properties in the final products. These include thermoplastic polymers, biodegradable materials, and specialized compounds with enhanced flow characteristics. Material selection is critical as it affects the flow behavior during the molding process, especially in micro-scale cavities. Advanced polymer blends and composites are developed to improve moldability, surface finish, and mechanical properties of micro-molded parts.Expand Specific Solutions03 Biomedical applications of microinjection molding
Microinjection molding has significant applications in the biomedical field. It is used to produce microfluidic devices, lab-on-a-chip systems, drug delivery components, and medical diagnostic tools. The technology enables the production of biocompatible micro-components with precise dimensions and surface features. These applications benefit from the ability to create complex geometries at the micro-scale while maintaining biocompatibility and sterility requirements.Expand Specific Solutions04 Process optimization and control in microinjection molding
Optimizing the microinjection molding process involves precise control of multiple parameters including injection speed, pressure, temperature, and cooling rate. Advanced monitoring systems and simulation tools are employed to predict and control material flow behavior at the micro-scale. Process optimization techniques focus on reducing cycle times, minimizing defects such as incomplete filling, and enhancing the replication accuracy of micro-features. These controls are essential for achieving consistent quality in high-volume production.Expand Specific Solutions05 Innovative mold designs and micro-feature fabrication
Novel mold designs and fabrication techniques are crucial for advancing microinjection molding capabilities. These include multi-cavity molds, inserts with micro-structured surfaces, and molds with integrated sensors. Advanced manufacturing methods such as micro-electrical discharge machining, laser ablation, and lithography-based techniques are used to create mold features at the micro-scale. These innovations enable the production of increasingly complex micro-components with enhanced surface details and tighter tolerances.Expand Specific Solutions
Leading Manufacturers and Research Institutions Analysis
Microinjection molding for medical applications is experiencing significant growth, currently transitioning from early adoption to mainstream implementation. The market is expanding rapidly, driven by increasing demand for miniaturized medical devices and components. Technologically, the field shows varying maturity levels across players. Academic institutions like Sichuan University and South China University of Technology are advancing fundamental research, while established manufacturers such as SABIC, IMA, and Fujitsu are developing commercial applications. Specialized companies like Continuus Pharmaceuticals and Nextbiomedical are creating innovative drug delivery systems. The competitive landscape features collaboration between research institutions (CNRS, INSERM) and industrial players, with companies like Taiwan Semiconductor bringing precision manufacturing expertise from adjacent industries to address the growing need for micro-scale medical components.
Continuus Pharmaceuticals, Inc.
Technical Solution: Continuus Pharmaceuticals has pioneered an Integrated Continuous Manufacturing (ICM) platform that incorporates micro-injection molding technologies for pharmaceutical applications. Their system enables the production of complex drug delivery devices with micro-features directly integrated into the continuous manufacturing process. The company's proprietary "Flow-Mold" technology allows for the creation of micro-structured components with feature sizes down to 5 micrometers while maintaining pharmaceutical-grade quality standards. Continuus has developed specialized polymer formulations optimized for micro-injection molding that are compatible with active pharmaceutical ingredients (APIs) and can withstand sterilization processes. Their platform includes in-line quality control systems using advanced imaging technologies to verify the dimensional accuracy of micro-molded components in real-time. The company has successfully implemented micro-injection molding for creating controlled-release drug delivery systems with precisely engineered microstructures that modulate drug release kinetics. Continuus has also developed micro-needle array technologies manufactured through their continuous micro-molding process for transdermal drug delivery applications.
Strengths: Seamless integration of micro-molding with pharmaceutical manufacturing processes; reduced time-to-market through continuous production capabilities; enhanced product consistency through automated quality control. Weaknesses: Regulatory complexity associated with novel manufacturing approaches; limited track record compared to established manufacturing methods; challenges in adapting the technology for diverse pharmaceutical applications.
I.M.A. Industria Macchine Automatiche SpA
Technical Solution: I.M.A. has developed advanced micro-injection molding systems specifically designed for medical and pharmaceutical applications. Their MINIJET platform incorporates high-precision micro-molding capabilities with automated assembly systems for the production of complex medical components. The company's technology enables the molding of components with wall thicknesses as low as 0.1mm and tolerances within ±0.01mm. I.M.A.'s micro-molding systems feature specialized hot runner systems optimized for medical-grade polymers, ensuring minimal material degradation and consistent part quality. Their proprietary "Clean-Mold" technology incorporates cleanroom-compatible molding processes with automated part extraction and handling to maintain sterility requirements for medical applications. I.M.A. has pioneered the integration of micro-sensors within the molding tools to provide real-time monitoring of critical process parameters, ensuring consistent quality of micro-molded medical components. The company has also developed specialized micro-mold surface treatments that enhance the release of complex geometries and improve the surface finish of medical components.
Strengths: Comprehensive integration of micro-molding with downstream assembly and packaging processes; extensive experience with GMP-compliant manufacturing systems; advanced process monitoring capabilities ensuring consistent quality. Weaknesses: Higher capital investment requirements compared to conventional molding systems; complex validation processes for pharmaceutical applications; specialized maintenance requirements for micro-molding equipment.
Key Patents and Breakthroughs in Medical Microinjection Technology
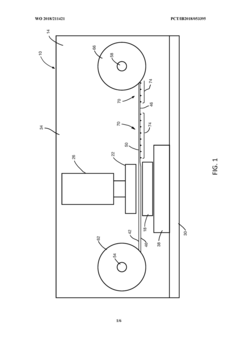
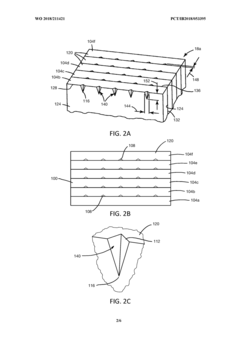
Methods and systems of producing polymer microneedle arrays via ultrasonic embossing, and resulting microneedle arrays
PatentWO2018211421A1
Innovation
- The use of ultrasonic embossing methods that allow gas to escape from cavities and control polymer temperature precisely, enabling the formation of solid microneedles with sharp tips and faster processing times, and a roll-to-roll approach for producing large arrays of microneedles.
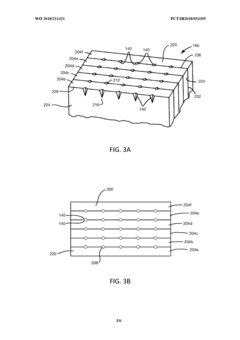
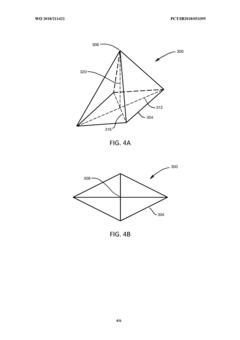
Array microinjection apparatuses and methods
PatentInactiveUS20130071873A1
Innovation
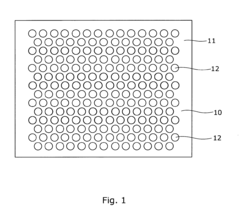
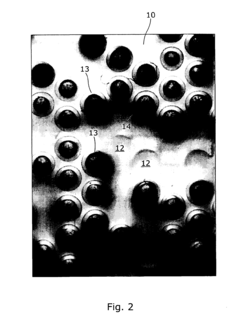
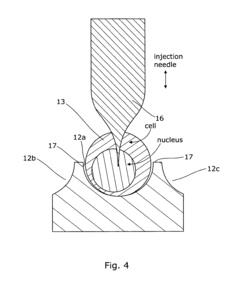
- An array microinjection apparatus with a substrate featuring a regular pattern of part-spherical recesses allows for precise location and simultaneous injection of cells or embryos, using a carriage with injectors to penetrate the cells or embryos in a controlled manner, facilitating accurate and efficient microinjection.
Regulatory Compliance and Standards for Medical Micro Components
The regulatory landscape for microinjection molded medical components is increasingly complex, with stringent requirements that manufacturers must navigate to ensure market access. The FDA in the United States, the EU Medical Device Regulation (MDR), and ISO standards collectively establish a comprehensive framework governing the production of micro-scale medical components. These regulations focus particularly on biocompatibility, sterilization validation, and material traceability—critical factors for components that often interface directly with human tissue or biological fluids.
ISO 10993 series standards have become the cornerstone for biocompatibility testing of medical micro components, requiring manufacturers to demonstrate that materials used in microinjection molding processes do not elicit adverse biological responses. This is complemented by ISO 13485, which establishes quality management systems specifically for medical device manufacturing, including specialized provisions for micro-scale production processes.
Recent regulatory developments have placed increased emphasis on process validation for microinjection molding. The FDA's guidance on process validation now requires manufacturers to demonstrate that their molding processes consistently produce components meeting predetermined specifications, with particular attention to dimensional accuracy at the micro scale. This has driven innovations in in-line inspection technologies and statistical process control methods tailored to microinjection molding operations.
Material master file submissions have also evolved to address the unique characteristics of polymers used in medical microinjection molding. Regulatory bodies now require comprehensive documentation of material properties at micro scales, where surface-to-volume ratios and molecular orientation can significantly differ from macro-scale applications. This has spurred collaboration between material suppliers and molders to develop specialized grades with enhanced documentation.
The emergence of combination products—incorporating both drug and device elements—has introduced additional regulatory complexity for microinjection molded components. These products must satisfy requirements from multiple regulatory pathways, necessitating early engagement with authorities and sophisticated design control processes that account for both pharmaceutical and device regulations.
International harmonization efforts, particularly through the Medical Device Single Audit Program (MDSAP), are gradually reducing regulatory fragmentation, allowing manufacturers of microinjection molded components to streamline compliance activities across major markets. However, regional differences in implementation timelines and technical requirements continue to present challenges for global product launches.
ISO 10993 series standards have become the cornerstone for biocompatibility testing of medical micro components, requiring manufacturers to demonstrate that materials used in microinjection molding processes do not elicit adverse biological responses. This is complemented by ISO 13485, which establishes quality management systems specifically for medical device manufacturing, including specialized provisions for micro-scale production processes.
Recent regulatory developments have placed increased emphasis on process validation for microinjection molding. The FDA's guidance on process validation now requires manufacturers to demonstrate that their molding processes consistently produce components meeting predetermined specifications, with particular attention to dimensional accuracy at the micro scale. This has driven innovations in in-line inspection technologies and statistical process control methods tailored to microinjection molding operations.
Material master file submissions have also evolved to address the unique characteristics of polymers used in medical microinjection molding. Regulatory bodies now require comprehensive documentation of material properties at micro scales, where surface-to-volume ratios and molecular orientation can significantly differ from macro-scale applications. This has spurred collaboration between material suppliers and molders to develop specialized grades with enhanced documentation.
The emergence of combination products—incorporating both drug and device elements—has introduced additional regulatory complexity for microinjection molded components. These products must satisfy requirements from multiple regulatory pathways, necessitating early engagement with authorities and sophisticated design control processes that account for both pharmaceutical and device regulations.
International harmonization efforts, particularly through the Medical Device Single Audit Program (MDSAP), are gradually reducing regulatory fragmentation, allowing manufacturers of microinjection molded components to streamline compliance activities across major markets. However, regional differences in implementation timelines and technical requirements continue to present challenges for global product launches.
Sustainability and Material Innovations in Medical Microinjection Molding
The medical microinjection molding industry is witnessing a significant shift toward sustainability, driven by increasing environmental regulations and growing consumer awareness. Manufacturers are actively exploring biodegradable polymers such as polylactic acid (PLA), polyhydroxyalkanoates (PHA), and polyglycolic acid (PGA) as alternatives to traditional petroleum-based plastics. These materials offer comparable mechanical properties while significantly reducing environmental impact through biodegradability and lower carbon footprints during production.
Recent innovations include the development of bio-based composites that combine natural fibers with biopolymers, creating materials with enhanced strength-to-weight ratios and reduced environmental impact. These composites are particularly valuable for implantable medical devices where biocompatibility and controlled degradation rates are essential performance parameters.
Closed-loop manufacturing systems are gaining traction in medical microinjection molding facilities, where production waste is recycled and reprocessed into new medical components. Advanced sorting and purification technologies ensure these recycled materials meet stringent medical-grade requirements, addressing previous concerns about contamination and material integrity.
Water-soluble support materials represent another sustainable innovation, allowing for complex geometries in medical components while eliminating the need for chemical solvents during post-processing. These materials dissolve in water after molding, reducing chemical waste and simplifying the manufacturing process for intricate medical devices.
Energy efficiency improvements in molding equipment have reduced the carbon footprint of microinjection molding operations. New-generation machines incorporate precision heating elements, improved insulation, and energy recovery systems that can reduce energy consumption by up to 50% compared to conventional equipment, while maintaining the precision required for medical components.
Material science advancements have also yielded antimicrobial polymers with inherent bacteria-resistant properties, eliminating the need for secondary antimicrobial treatments. These materials incorporate silver ions or organic antimicrobial compounds directly into the polymer matrix, providing long-lasting protection against healthcare-associated infections without additional manufacturing steps.
The integration of nanotechnology has enabled the development of nanocomposite materials with enhanced properties such as improved barrier characteristics, mechanical strength, and thermal stability at lower material volumes. This allows for thinner-walled components that require less raw material while maintaining or exceeding performance specifications, contributing to both sustainability goals and miniaturization trends in medical devices.
Recent innovations include the development of bio-based composites that combine natural fibers with biopolymers, creating materials with enhanced strength-to-weight ratios and reduced environmental impact. These composites are particularly valuable for implantable medical devices where biocompatibility and controlled degradation rates are essential performance parameters.
Closed-loop manufacturing systems are gaining traction in medical microinjection molding facilities, where production waste is recycled and reprocessed into new medical components. Advanced sorting and purification technologies ensure these recycled materials meet stringent medical-grade requirements, addressing previous concerns about contamination and material integrity.
Water-soluble support materials represent another sustainable innovation, allowing for complex geometries in medical components while eliminating the need for chemical solvents during post-processing. These materials dissolve in water after molding, reducing chemical waste and simplifying the manufacturing process for intricate medical devices.
Energy efficiency improvements in molding equipment have reduced the carbon footprint of microinjection molding operations. New-generation machines incorporate precision heating elements, improved insulation, and energy recovery systems that can reduce energy consumption by up to 50% compared to conventional equipment, while maintaining the precision required for medical components.
Material science advancements have also yielded antimicrobial polymers with inherent bacteria-resistant properties, eliminating the need for secondary antimicrobial treatments. These materials incorporate silver ions or organic antimicrobial compounds directly into the polymer matrix, providing long-lasting protection against healthcare-associated infections without additional manufacturing steps.
The integration of nanotechnology has enabled the development of nanocomposite materials with enhanced properties such as improved barrier characteristics, mechanical strength, and thermal stability at lower material volumes. This allows for thinner-walled components that require less raw material while maintaining or exceeding performance specifications, contributing to both sustainability goals and miniaturization trends in medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!