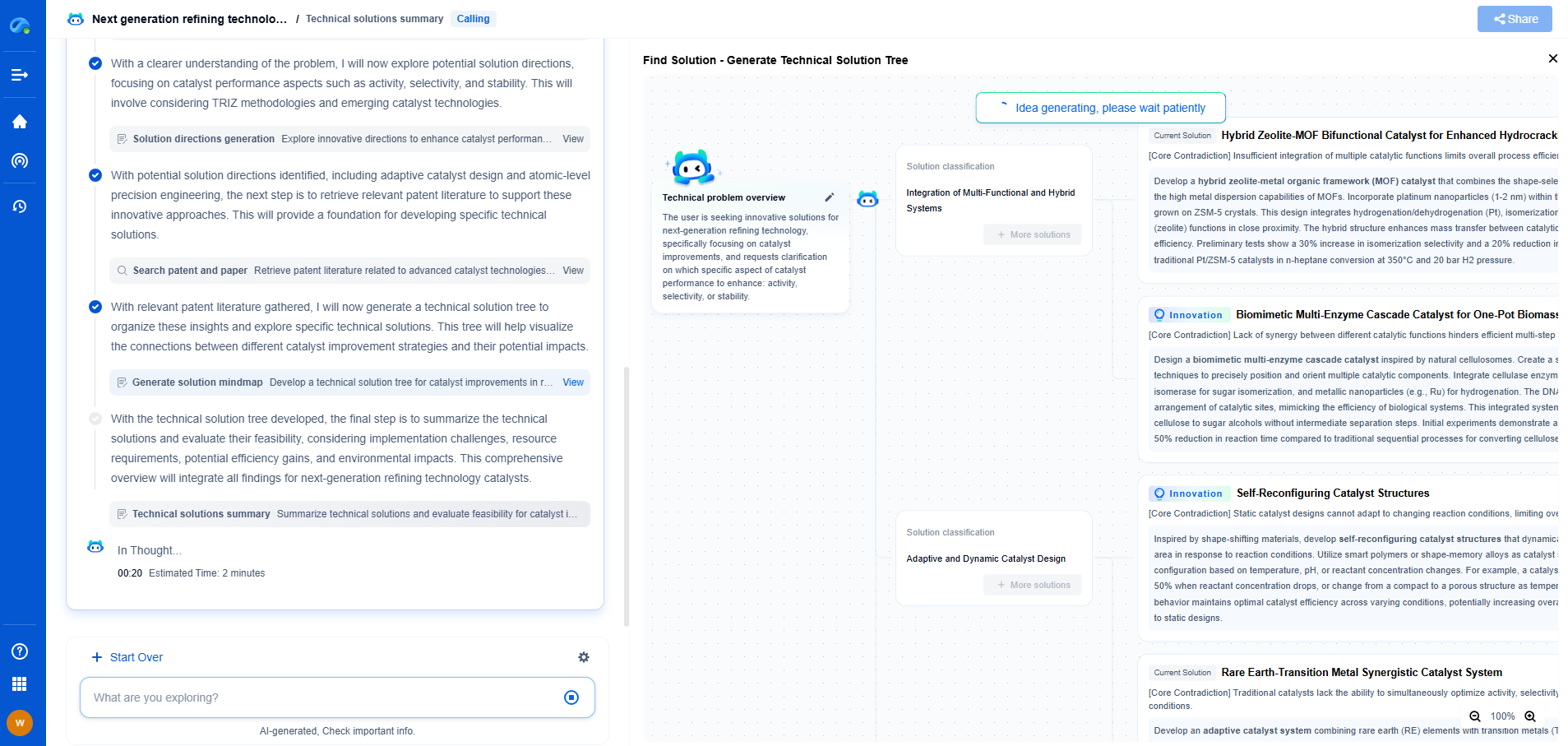
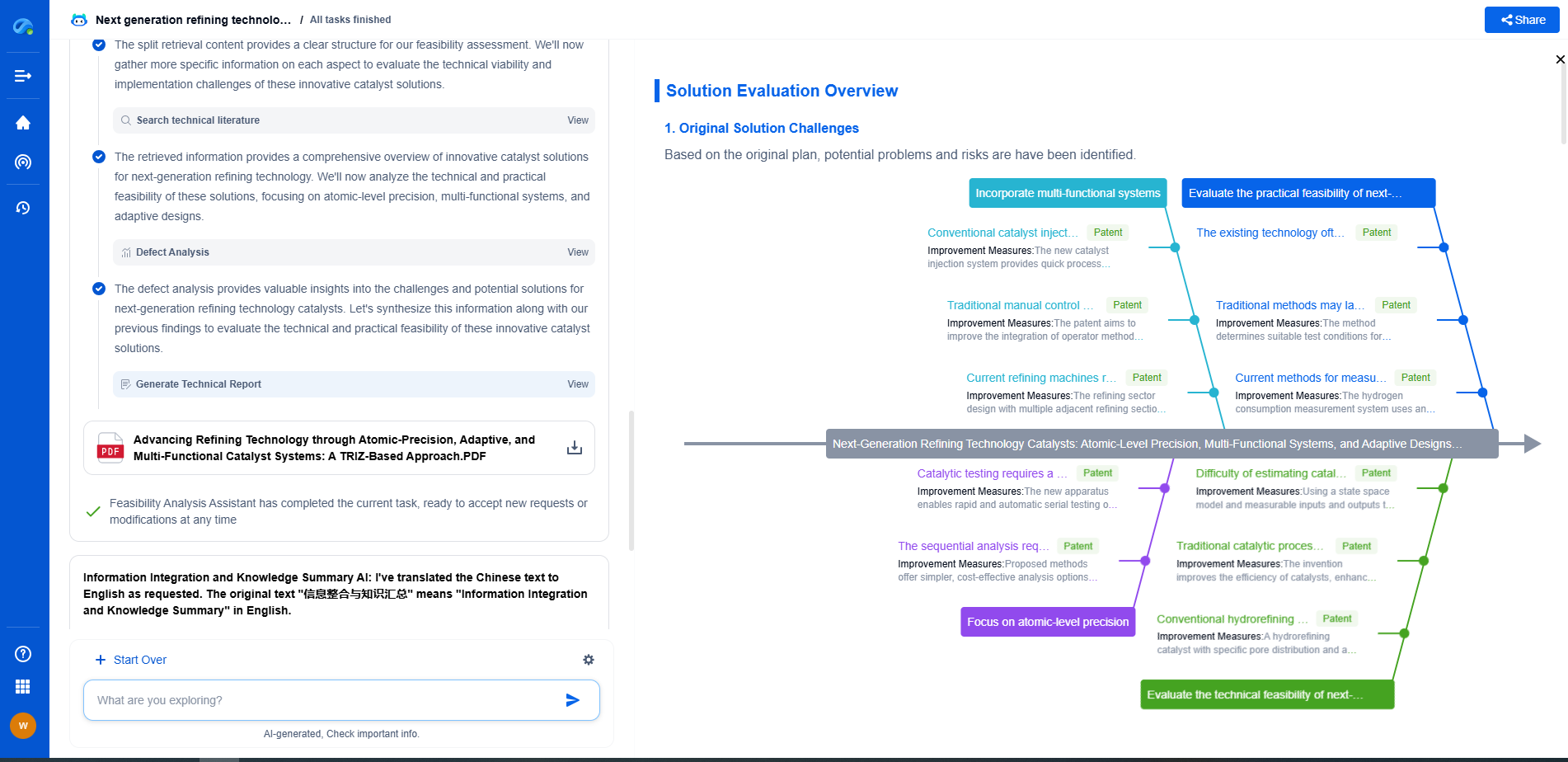
ASME BPE: Bioprocessing Equipment Standards for Filtration
JUL 25, 2025 |
The American Society of Mechanical Engineers (ASME) Bioprocessing Equipment (BPE) standards are vital in the design and manufacturing of equipment used in the bioprocessing industry. These standards, particularly in filtration, ensure the safety, quality, and efficacy of biopharmaceutical products. In this blog, we explore the significance of ASME BPE standards and their application in filtration processes.
The Importance of Filtration in Bioprocessing
Filtration is a critical process in bioprocessing, used to remove impurities and ensure the purity of end products. It involves separating particles and microbial contaminants from liquids or gases, which is crucial for maintaining product quality and safety. Effective filtration impacts the overall performance and efficiency of bioprocessing systems, making adherence to standards essential.
ASME BPE's Role in Filtration Standards
ASME BPE standards provide comprehensive guidelines for the design, construction, and testing of bioprocessing equipment, including filtration systems. These standards ensure that equipment meets stringent requirements for performance, safety, and hygiene. By adhering to ASME BPE guidelines, manufacturers can optimize their filtration systems to meet industry demands and regulatory requirements.
Design Considerations for Filtration Equipment
When designing filtration systems under ASME BPE standards, engineers must consider factors such as material compatibility, surface finish, and cleanability. Materials used in filter systems must resist corrosion and withstand repeated cleaning and sterilization processes. Surface finishes should be smooth to prevent microbial growth and ensure efficient cleaning. The design must also facilitate easy disassembly and reassembly for maintenance and validation.
Testing and Validation Protocols
ASME BPE standards emphasize rigorous testing and validation protocols to ensure filtration systems perform as intended. This includes integrity testing, pressure decay tests, and bacterial retention challenges. These tests verify that the filtration systems effectively remove contaminants and operate within specified parameters. Regular validation ensures continued compliance with ASME BPE standards and regulatory requirements.
Impact of ASME BPE Standards on the Industry
The adoption of ASME BPE standards has significantly impacted the bioprocessing industry by enhancing equipment reliability and product quality. These standards facilitate global harmonization, allowing companies to compete in international markets by ensuring their equipment meets universally recognized quality benchmarks. Compliance with ASME BPE standards also minimizes the risk of product recalls and regulatory penalties.
Challenges and Future Directions
Despite the benefits, challenges exist in implementing ASME BPE standards, particularly for companies with legacy systems. Upgrading or replacing equipment to meet current standards can be costly and time-consuming. However, the long-term benefits of compliance, including improved efficiency and reduced risk, often outweigh the initial investment.
Looking forward, ASME BPE standards will continue to evolve to address emerging technologies and industry trends. The ongoing development of these standards will ensure they remain relevant and continue to support innovation and excellence in bioprocessing.
Conclusion
ASME BPE standards play a critical role in ensuring the quality and safety of bioprocessing equipment, particularly in filtration processes. By adhering to these standards, manufacturers can enhance the reliability and performance of their systems, ultimately contributing to the production of high-quality biopharmaceutical products. As the industry advances, continual adherence to and evolution of ASME BPE standards will be crucial for maintaining the integrity and competitiveness of bioprocessing operations.
From next-generation membrane materials to high-efficiency separation processes for pharmaceuticals, water treatment, food processing, or energy systems, the filtration & separation industry is rapidly evolving with a surge in material innovation, microstructure design, and process optimization.
Patsnap Eureka, our intelligent AI assistant built for R&D professionals in high-tech sectors, empowers you with real-time expert-level analysis, technology roadmap exploration, and strategic mapping of core patents—all within a seamless, user-friendly interface.
Whether you're designing the next high-throughput filter, optimizing nanostructured surfaces, or exploring new separation media for emerging industries—Patsnap Eureka gives you AI-driven insights in seconds, helping you move from ideation to innovation with confidence.
🚀 Start your free trial today and experience how Eureka transforms filtration innovation—from reactive to predictive.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com