Classifying Defects: Random vs Systematic Patterns
JUL 8, 2025 |
In any system, whether it's manufacturing, software development, or even natural processes, defects are inevitable. Understanding these defects is crucial for improving quality and efficiency. Defects can generally be classified into two broad categories: random and systematic. Each type has distinct characteristics, causes, and implications for how they should be addressed. Understanding the differences between these two types of defects is essential for any organization aiming to enhance its processes and products.
What Are Random Defects?
Random defects, as the name suggests, occur without a predictable pattern. They are sporadic and are typically caused by external factors that are not easily controlled. For example, in a manufacturing context, random defects might occur due to variations in raw materials, unexpected machine malfunctions, or human errors that happen occasionally. Random defects can also be attributed to unforeseen environmental conditions or user interactions in software systems.
Addressing random defects is challenging because they don't follow a consistent pattern. Instead of focusing on specific issues, organizations need to implement broad quality control measures. This may include regular equipment maintenance, comprehensive training programs for employees, and a robust feedback loop to identify and address issues as they arise. Additionally, statistical quality control methods, such as control charts, can help identify random variations and keep them in check.
Systematic Defects: A Deeper Dive
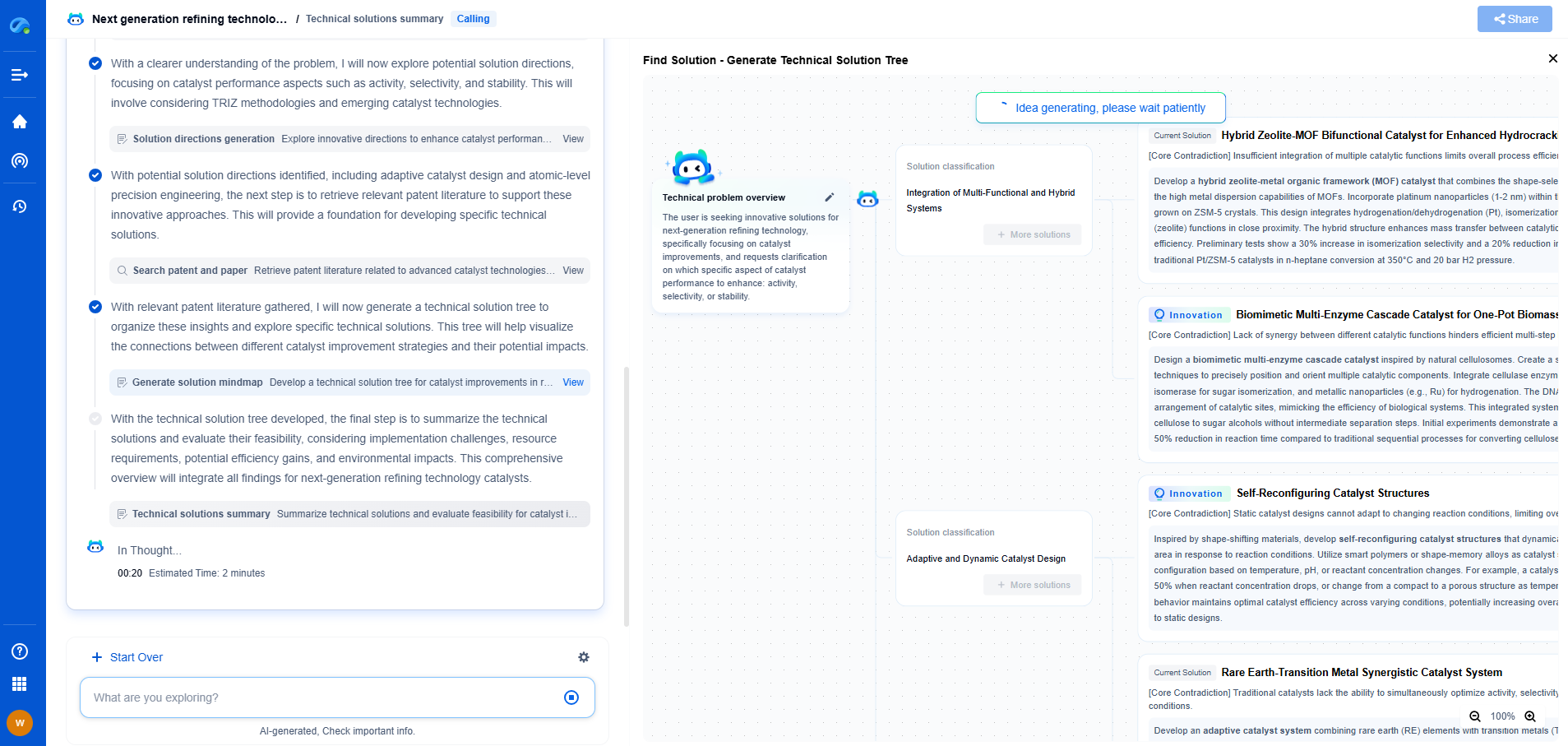
Systematic defects, unlike random ones, have a predictable pattern and are often ingrained within the system. These defects arise from inherent issues in the design, process, or material that consistently lead to failures. For example, a systematic defect in software could be a flaw in the algorithm that causes errors under specific conditions. In manufacturing, it might be a misaligned component in a production line that consistently produces defective products.
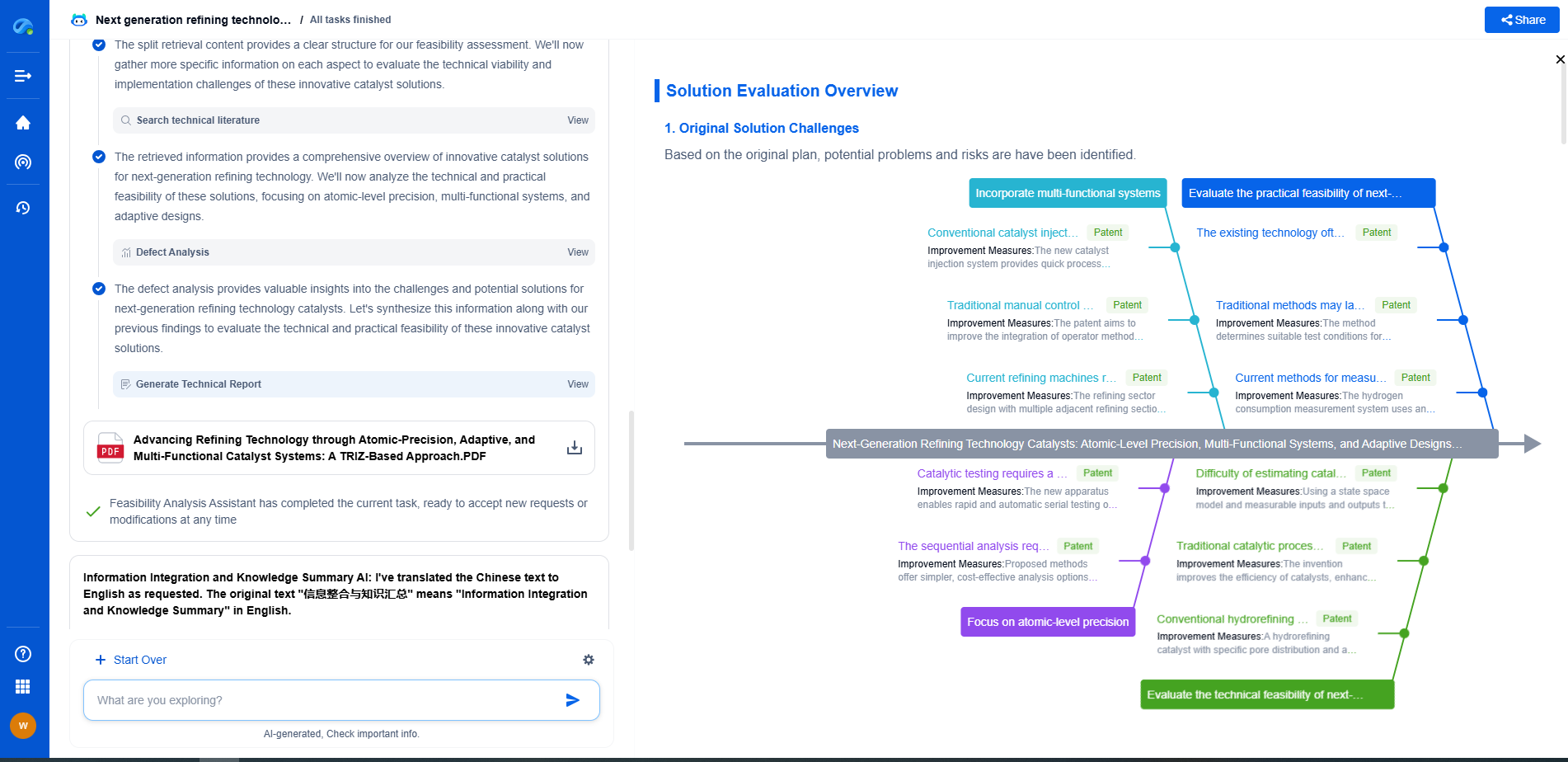
The key to addressing systematic defects lies in identifying the root cause of the problem. This often involves a detailed analysis of the entire process or system to pinpoint where the defect originates. Techniques such as root cause analysis, Six Sigma methodologies, and failure mode and effects analysis (FMEA) are commonly used to uncover and address these defects. By focusing on the underlying issues, organizations can implement targeted improvements that eliminate the defect at its source.
Implications for Quality Control and Improvement
The distinction between random and systematic defects has significant implications for quality control and improvement strategies. Random defects require a broad approach that emphasizes flexibility and adaptability. Systems should be designed with contingencies and redundancies to handle unforeseen variations. Regular audits and testing can help identify and mitigate random defects before they escalate.
On the other hand, systematic defects require a more focused and analytical approach. By understanding the root causes, organizations can implement precise changes that lead to long-term improvements. This often involves redesigning processes, retraining staff, or updating systems to eliminate the conditions that lead to defects.
The Role of Data in Defect Classification
Data plays a crucial role in distinguishing between random and systematic defects. By collecting and analyzing data on defects over time, organizations can identify patterns and trends that indicate the nature of the defects they're facing. Statistical tools and software can help highlight anomalies and consistent issues, guiding decision-makers in their quality improvement efforts.
The integration of data analytics and machine learning in defect classification is becoming increasingly common. These technologies can process vast amounts of data quickly, identifying subtle patterns that may not be immediately apparent to human analysts. As a result, organizations can gain deeper insights into the nature of their defects and tailor their strategies accordingly.
Conclusion: A Balanced Approach
In conclusion, understanding the difference between random and systematic defects is crucial for any organization looking to improve its processes and products. While random defects require flexible and broad strategies to manage unpredictability, systematic defects demand a focused approach aimed at root cause elimination. By leveraging data and modern analytical tools, organizations can gain a better understanding of their defect landscape and implement more effective quality control measures. This balanced approach ultimately leads to enhanced product quality, improved customer satisfaction, and a more efficient operation overall.
Infuse Insights into Chip R&D with PatSnap Eureka
Whether you're exploring novel transistor architectures, monitoring global IP filings in advanced packaging, or optimizing your semiconductor innovation roadmap—Patsnap Eureka empowers you with AI-driven insights tailored to the pace and complexity of modern chip development.
Patsnap Eureka, our intelligent AI assistant built for R&D professionals in high-tech sectors, empowers you with real-time expert-level analysis, technology roadmap exploration, and strategic mapping of core patents—all within a seamless, user-friendly interface.
👉 Join the new era of semiconductor R&D. Try Patsnap Eureka today and experience the future of innovation intelligence.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com