Comparing Conductivity: Copper vs Aluminum vs Silver
JUN 27, 2025 |
Conductivity is a fundamental property of materials that indicates their ability to conduct electric current. This property is crucial for various applications, particularly in electrical engineering, electronics, and materials science. When it comes to metals, copper, aluminum, and silver are among the top contenders known for their excellent electrical conductivity. Each of these metals possesses unique characteristics that make them suitable for different applications. This article delves into the comparative analysis of copper, aluminum, and silver in terms of their conductivity and explores their benefits and limitations.
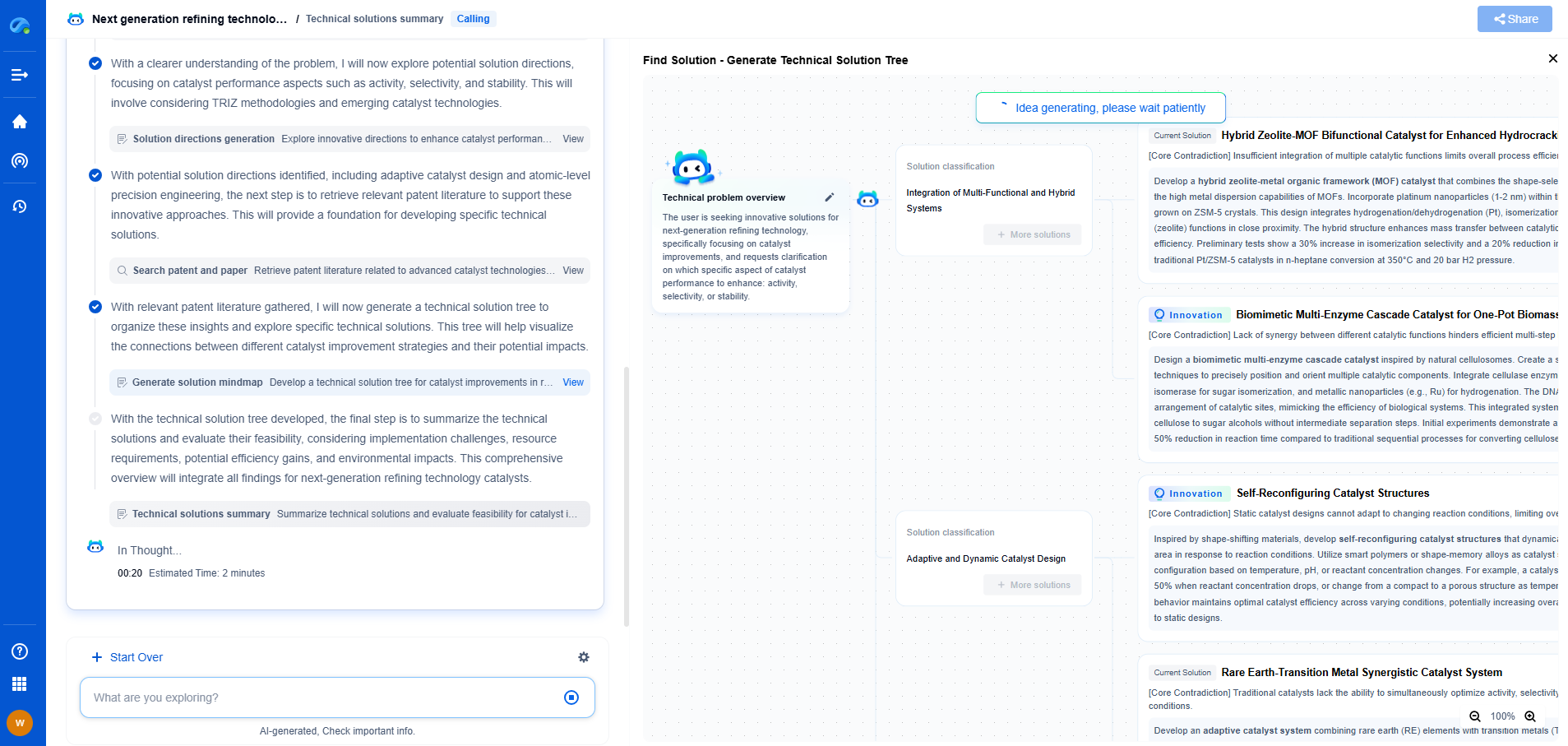
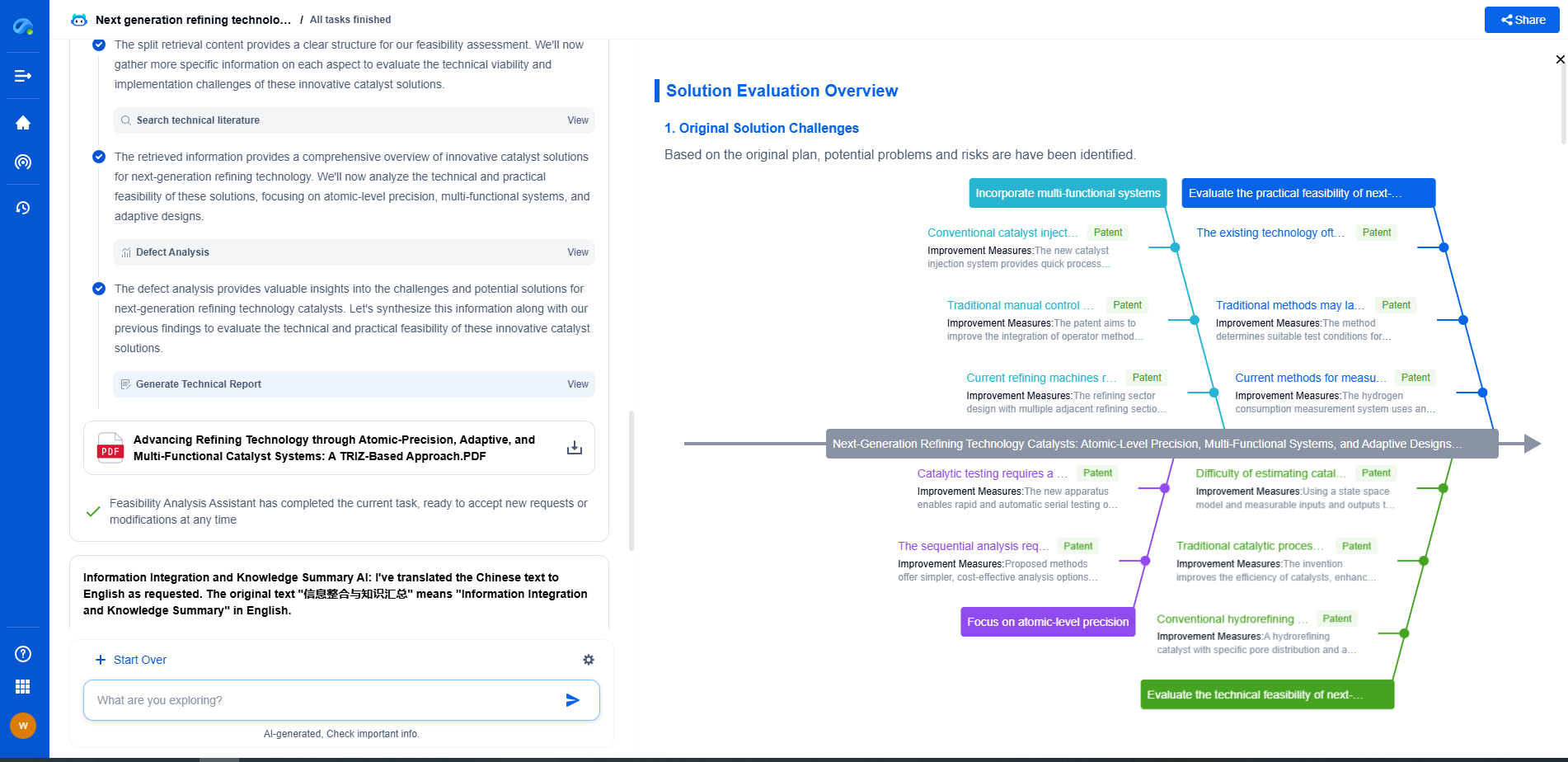
**Copper: The Standard for Electrical Conductivity**
Copper is often considered the standard when it comes to electrical conductivity due to its excellent conductive properties. With a conductivity value of approximately 5.96 x 10^7 S/m, copper is widely used in electrical wiring, motors, and numerous industrial applications. Its high conductivity, coupled with favorable mechanical properties such as ductility and corrosion resistance, makes it an ideal choice for many uses.
Beyond its electrical capabilities, copper's thermal conductivity is also noteworthy, making it a preferred choice for heat exchangers and refrigeration systems. However, its higher cost compared to aluminum and its heavier weight can be drawbacks in certain applications where cost-effectiveness and lightweight materials are priorities.
**Aluminum: Lightweight and Cost-Effective**
Aluminum offers a unique blend of characteristics that make it highly competitive in specific contexts. While its conductivity, at about 3.5 x 10^7 S/m, is lower than that of copper, aluminum's lightweight nature makes it advantageous for applications where weight is a critical factor. This characteristic is particularly beneficial in the aerospace and automotive industries, where every gram counts.
Moreover, aluminum is significantly more cost-effective than copper, which can lead to substantial savings in large-scale projects. Despite these benefits, aluminum does present some challenges. Its lower conductivity means that thicker wires are necessary to carry the same current as copper, which can affect space and design constraints. Additionally, aluminum is more prone to oxidation and requires careful handling to prevent long-term degradation.
**Silver: The Best Conductor, At a Price**
Silver holds the crown for electrical conductivity with a value of 6.30 x 10^7 S/m, higher than both copper and aluminum. Its unparalleled conductivity makes it ideal for specialized applications where performance is paramount, such as in high-frequency conductors and precision instruments.
Despite its superior conductivity, silver's practical use is limited by its high cost. The expense of silver makes it impractical for general applications, and it is typically reserved for high-end or critical components where its benefits outweigh its price. Additionally, silver tarnishes over time, which can affect its conductivity. This requires additional maintenance to ensure optimal performance.
**Comparative Analysis**
When comparing copper, aluminum, and silver, it is essential to consider the specific requirements of the intended application. Copper is a versatile all-rounder with excellent conductivity and mechanical properties, making it suitable for a wide range of applications. Aluminum is a cost-effective, lightweight alternative, ideal for weight-sensitive projects. Silver, while the best conductor, is reserved for specific, high-performance requirements due to its cost.
**Applications and Decision-Making**
Choosing between copper, aluminum, and silver requires a thorough understanding of the specific needs of the project. For general electrical wiring and infrastructure, copper remains the go-to choice due to its balance of conductivity, durability, and cost. In contrast, aluminum is favored in environments where weight reduction is critical and budget constraints are stringent. Silver, with its exceptional conductivity, is selected for niche applications where its cost can be justified by the performance benefits it provides.
**Conclusion**
In the realm of electrical conductivity, each metal offers its own set of advantages and limitations. Understanding these differences is key to optimizing material selection for specific applications. Whether prioritizing conductivity, cost, weight, or performance, the choice between copper, aluminum, and silver depends on balancing these factors to achieve the desired outcome. By carefully evaluating the properties and applications of these metals, engineers and designers can make informed decisions that best suit their project's needs.
Empower Your Breakthroughs in Basic Electric Components with Patsnap Eureka
From resistors, capacitors, and inductors to fuses, connectors, superconductors, and nano-scale materials—basic electric elements may be the building blocks of modern electronics, but the innovation behind them is anything but simple. As device miniaturization accelerates and materials science pushes new frontiers, R&D and IP teams face increasing complexity in staying on top of technical advancements, patent activity, and competitive landscapes.
Patsnap Eureka, our intelligent AI assistant built for R&D professionals in high-tech sectors, empowers you with real-time expert-level analysis, technology roadmap exploration, and strategic mapping of core patents—all within a seamless, user-friendly interface.
🔧 Whether you’re optimizing energy storage, improving thermal resistance, or creating the next leap in circuit efficiency, Patsnap Eureka is your AI copilot for high-efficiency, high-precision R&D and IP strategy.
👉 Experience how Patsnap Eureka can revolutionize your R&D and IP strategy. Request a demo today and power up your next breakthrough.
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com